All Photos(1)
About This Item
Empirical Formula (Hill Notation):
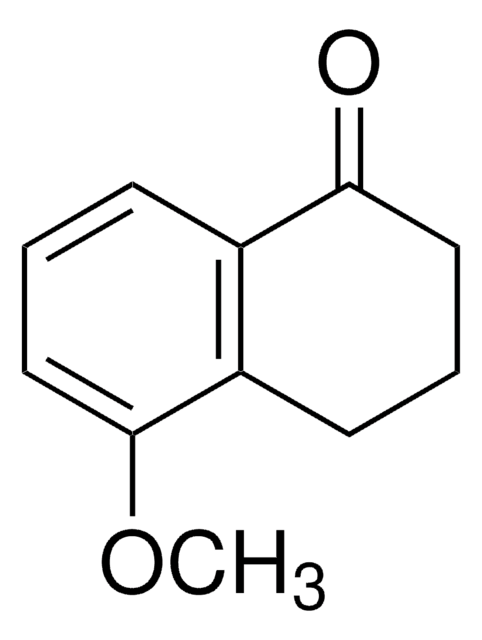
C10H10O2
CAS Number:
Molecular Weight:
162.19
Beilstein:
2437410
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
mp
206-209 °C (lit.)
functional group
ketone
SMILES string
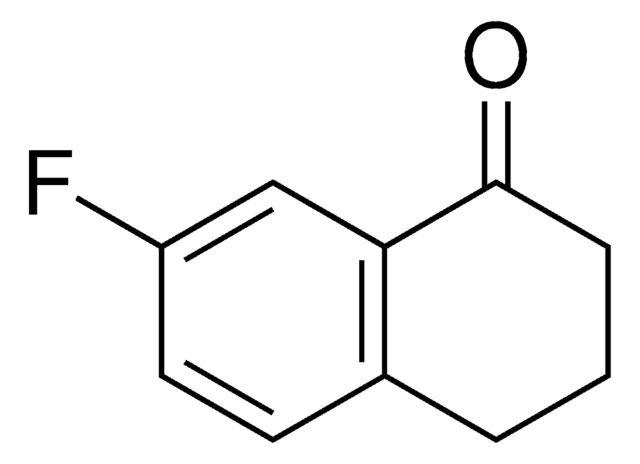
Oc1cccc2C(=O)CCCc12
InChI
1S/C10H10O2/c11-9-5-1-3-7-8(9)4-2-6-10(7)12/h1,3,5,11H,2,4,6H2
InChI key
YPPZCRZRQHFRBH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
5-Hydroxy-1-tetralone was used:
- as internal standard during HPLC determination of 4-hydroxymephenytoin (4-OH-M) in human urine
- as fluorescent labeling reagent during microdetection of glycosphingolipid on TLC plates
- in synthesis of 1,2,3,4-tetrahydro-5H-1-benzazepine-quinone derivatives
- in synthesis of new chiral oxathiane
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Studies on Quinones. Part 22.'Synthesis of 1-Benzazepine-6, 9-Quinone Derivatives.
Valderrama JA, et al.
Synthetic Communications, 22(4), 629-640 (1992)
K Watanabe et al.
Journal of lipid research, 36(8), 1848-1855 (1995-08-01)
A microdetection system for glycosphingolipid analysis has been developed using 5-hydroxy-1-tetralone as the fluorescent labeling reagent. The reagents in H2SO4 permit the fluorometric detection of acidic and neutral glycosphingolipids both in test tube and on thin-layer chromatographic plates. Glycosphingolipids can
A new chiral oxathiane: synthesis, resolution and absolute configuration determination by vibrational circular dichroism.
Solladie-Cavallo A, et al.
Tetrahedron Asymmetry, 12(18), 2605-2611 (2001)
M Tanaka et al.
Journal of chromatography. B, Biomedical applications, 676(1), 87-94 (1996-02-09)
A simple and selective HPLC method for the determination of 4-hydroxymephenytoin (4-OH-M) in human urine, using a controlled potential coulometric detector equipped with a dual working electrode cell of fully porous graphite, has been developed. After acid hydrolysis of urine
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service