212830
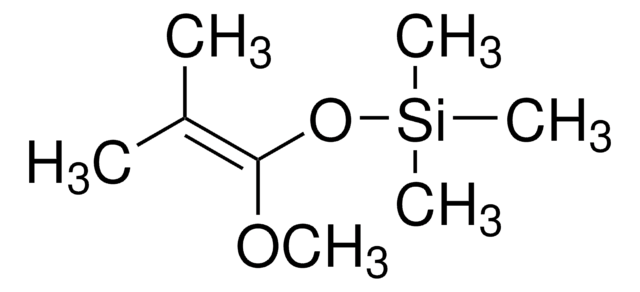
trans-1-Methoxy-3-trimethylsiloxy-1,3-butadiene
95%
Synonym(s):
Danishefsky’s diene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)3SiOC(=CH2)CH=CHOCH3
CAS Number:
Molecular Weight:
172.30
Beilstein:
1616761
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
form
liquid
impurities
2-5% 4-methoxy-3-buten-2-one
refractive index
n20/D 1.454 (lit.)
bp
68-69 °C/14 mmHg (lit.)
density
0.885 g/mL at 25 °C (lit.)
functional group
ether
storage temp.
2-8°C
SMILES string
CO\C=C\C(=C)O[Si](C)(C)C
InChI
1S/C8H16O2Si/c1-8(6-7-9-2)10-11(3,4)5/h6-7H,1H2,2-5H3/b7-6+
InChI key
SHALBPKEGDBVKK-VOTSOKGWSA-N
Looking for similar products? Visit Product Comparison Guide
General description
trans-1-methoxy-3-trimethylsiloxy-1,3-butadiene is a funtionalized Diels-Alder diene. Mukaiyama-Michael-type addition/heterocyclization of trans-1-methoxy-3-trimethylsiloxy-1,3-butadiene (Danishefsky′s diene) with 1,2-diaza-1,3-butadiene has been investigated. Asymmetric hetero-Diels-Alder cyclization of Danishefsky′s diene with benzaldehyde catalyzed by mesoporous inorganic/metalorganic hybrid materials has been reported.
Application
trans-1-Methoxy-3-trimethylsiloxy-1,3-butadiene was used:
- in the synthesis of sulfone analogues of griseofulvin (sulfogriseofulvins), 4H-1-aminopyrroles and 4,5H-pyrazoles
- as Diels-Alder diene for the synthesis of pyridones and pyranones
- as reagent employed in the Mannich-Michael reaction for preparation of piperidinones and enaminones
accessory
Product No.
Description
Pricing
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
138.2 °F - closed cup
Flash Point(C)
59 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
M Friedrich et al.
Archiv der Pharmazie, 329(7), 361-370 (1996-07-01)
Syntheses of substituted, especially of fluoro substituted benzoxathiole 1,1-dioxides, are described. These derivatives were transformed via the Peterson olefination into substituted 2-alkylidene derivatives 27. Diels-Alder reactions of 27 with 1,1-dimethoxy- and 1-methoxy-3-trimethylsiloxy-1,3-butadiene (30, 32) gave sulfone analogues 31 of griseofulvin
Tetrahedron, 49, 397-397 (1993)
The Journal of Organic Chemistry, 57, 4444-4444 (1992)
The Journal of Organic Chemistry, 57, 3605-3605 (1992)
Tetrahedron, 49, 1749-1749 (1993)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service