192333
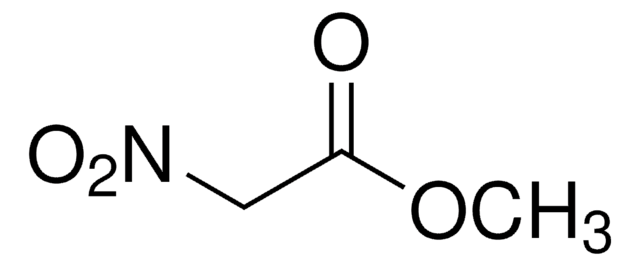
Ethyl nitroacetate
97%
Synonym(s):
2-Nitroacetic acid ethyl ester, Ethyl 2-nitroacetate, Nitroacetic acid ethyl ester
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
NO2CH2CO2C2H5
CAS Number:
Molecular Weight:
133.10
Beilstein:
1210027
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.424 (lit.)
bp
105-107 °C/25 mmHg (lit.)
density
1.199 g/mL at 25 °C (lit.)
functional group
amine
ester
nitro
SMILES string
CCOC(=O)C[N+]([O-])=O
InChI
1S/C4H7NO4/c1-2-9-4(6)3-5(7)8/h2-3H2,1H3
InChI key
FTKASJMIPSSXBP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Ethyl nitroacetate has been used in:
- synthesis of γ-oxoacids via Michael addition reaction with α,β-unsaturated ketones
- fuctionalization of C4-position on pyrimidine and C6-position on 2′-deoxyguanosine to produce novel nucleosides
- facile synthesis of α,α-diisobutylglycine
- synthesis of DL-4,4-difluoroglutamic acid
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
197.6 °F - closed cup
Flash Point(C)
92 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yanwen Fu et al.
The Journal of organic chemistry, 68(25), 9854-9857 (2003-12-06)
alpha,alpha-Diisobutylglycine has been synthesized using a Pd-mediated dialkylation of ethyl nitroacetate as a key first step. The free alphaalphaAA is N(alpha)-protected and has been applied to the assembly of conformationally constrained peptide analogues. Mixed anhydrides from BOP-Cl and Fmoc-alphaalphaAA-OH are
Victor Timoshchuk
Nucleosides, nucleotides & nucleic acids, 24(5-7), 1043-1046 (2005-10-27)
A study of C-nucleophilic substitution at the C4-position on pyrimidine and C6-position on 2'-deoxyguanosine to produce novel nucleosides is presented with the spectroscopic properties of their respective substitution products. C4-(1,2,4-triazol-1-yl) pyrimidine nucleosides 1 were treated with nitroalkanes, malononitrile, acetylacetone, ethyl
Elena Trogu et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 18(7), 2081-2093 (2012-01-12)
Base-catalysed condensation reactions of nitroacetic esters with dipolarophiles to give isoxazole derivatives proceed faster, and often with higher yields, in the presence of water than in organic solvents such as chloroform. Kinetic profiles show that induction times are greatly reduced
Maialen Aginagalde et al.
The Journal of organic chemistry, 75(21), 7435-7438 (2010-10-05)
Michael addition of ethyl nitroacetate on α,β-unsaturated ketones followed by Nef oxidation under hydrolytic conditions yields γ-oxoacids instead of the corresponding α,δ-dioxoesters. A concerted decarboxylation step is proposed on the basis of computational results. Finally, conversion of the γ-ketoacids thus
Indranil Bhattacharjee et al.
Physical chemistry chemical physics : PCCP, 20(9), 6060-6072 (2017-12-23)
Achieving synthetic control over light-driven molecular dynamics is essential for designing complex molecule-based devices. Here we design a novel coumarin-imidazole conjugate (1) whose excited state structural dynamics are primarily controlled by a distant intramolecular H-bonding interaction within the backbone. The
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service