All Photos(1)
About This Item
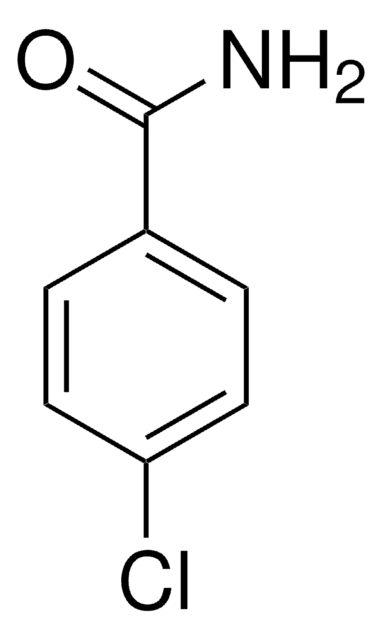
Linear Formula:
BrC6H4CONH2
CAS Number:
Molecular Weight:
200.03
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
powder
mp
190-193 °C (lit.)
functional group
amide
SMILES string
NC(=O)c1ccc(Br)cc1
InChI
1S/C7H6BrNO/c8-6-3-1-5(2-4-6)7(9)10/h1-4H,(H2,9,10)
InChI key
ZRWNRAJCPNLYAK-UHFFFAOYSA-N
Related Categories
General description
Electrochemical fluorination of 4-bromobenzamide in anhydrous HF has been investigated.
Application
4-Bromobenzamide was used in the preparation of (N-benzylpiperidin-4-yl)-4-tri-n-butylstannylbenzamide.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
C S John et al.
Cancer research, 55(14), 3022-3027 (1995-07-15)
The synthesis of [125I]-N-(N-benzylpiperidin-4-yl)-4-iodobenzamide (4-[125I]BP), a novel radiopharmaceutical that possesses high affinity for both sigma-1 and sigma-2 receptor subtypes, and its binding characteristics to MCF-7 breast cancer cells are described. To obtain high yields (with high specific activity) of radioiodinated
Electrochemical fluorination of aromatic compounds in anhydrous HF.
Shainyan BA and Danilevich YS.
Russ. J. Org. Chem., 42(2), 214-219 (2006)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![9-Borabicyclo[3.3.1]nonane solution 0.5 M in THF](/deepweb/assets/sigmaaldrich/product/structures/180/891/8b64e597-269d-4780-98b6-40889dfd06b9/640/8b64e597-269d-4780-98b6-40889dfd06b9.png)


![9-Borabicyclo[3.3.1]nonane dimer](/deepweb/assets/sigmaaldrich/product/structures/203/431/624973a6-aec1-4b23-b6c4-013285ac418c/640/624973a6-aec1-4b23-b6c4-013285ac418c.png)