All Photos(2)
About This Item
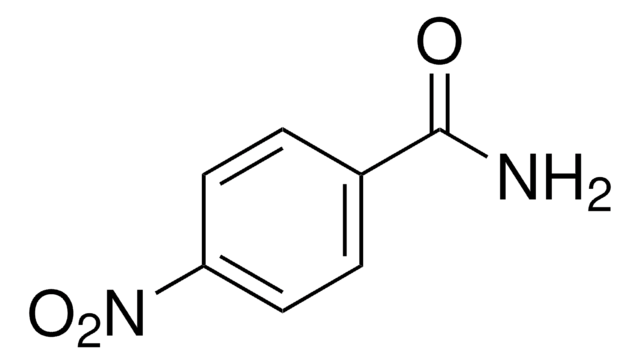
Linear Formula:
O2NC6H4CONH2
CAS Number:
Molecular Weight:
166.13
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
140-143 °C (lit.)
functional group
amide
nitro
SMILES string
NC(=O)c1cccc(c1)[N+]([O-])=O
InChI
1S/C7H6N2O3/c8-7(10)5-2-1-3-6(4-5)9(11)12/h1-4H,(H2,8,10)
InChI key
KWAYEPXDGHYGRW-UHFFFAOYSA-N
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
M R Purnell et al.
The Biochemical journal, 185(3), 775-777 (1980-03-01)
In a search for new inhibitors of the nuclear enzyme poly(ADP-ribose) synthetase, it was found that various benzamides substituted in the 3-position were the most inhibitory compounds found to date. Two of the benzamides, 3-aminobenzamide and 3-methoxybenzamide, were found to
Y Uchigata et al.
The Journal of biological chemistry, 257(11), 6084-6088 (1982-06-10)
We have shown previously that alloxan and streptozotocin, two major diabetogenic agents, cause DNA strand breaks in rat pancreatic islets and stimulate nuclear poly(ADP-ribose) synthetase, thereby depleting intracellular NAD level and inhibiting proinsulin synthesis (Okamoto, H. (1981) Mol. Cell. Biochem.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service