All Photos(2)
About This Item
Linear Formula:
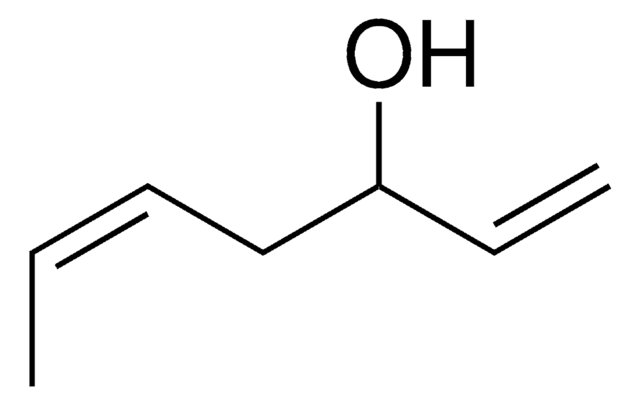
CH3CH(C6H5)CH2CH2OH
CAS Number:
Molecular Weight:
150.22
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.52 (lit.)
bp
138-140 °C/33 mmHg (lit.)
density
0.972 g/mL at 25 °C (lit.)
functional group
hydroxyl
phenyl
SMILES string
CC(CCO)c1ccccc1
InChI
1S/C10H14O/c1-9(7-8-11)10-5-3-2-4-6-10/h2-6,9,11H,7-8H2,1H3
InChI key
SQGBBDFDRHDJCJ-UHFFFAOYSA-N
General description
3-Phenyl-1-butanol undergoes biotransformation to aldehydes by oxidation in the presence of Gluconobacteroxydans DSM 2343. It undergoes enantioselective transesterification reaction with vinyl acetate catalyzed by lipase isolated from Pseudomonas cepacia.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Chemoselective oxidation of primary alcohols to aldehydes with Gluconobacter oxydans.
Villa R, et al.
Tetrahedron Letters, 43(34), 6059-6061 (2002)
The effect of vinyl esters on the enantioselectivity of the lipase-catalysed transesterification of alcohols.
Kawasaki M, et al.
Tetrahedron Asymmetry, 12(4), 585-596 (2001)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service