186708
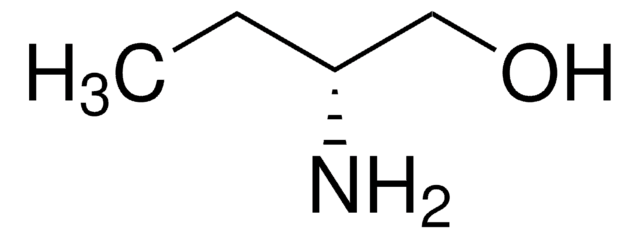
(S)-(+)-2-Amino-3-methyl-1-butanol
96%
Synonym(s):
L-Valinol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)2CHCH(NH2)CH2OH
CAS Number:
Molecular Weight:
103.16
Beilstein:
1719137
EC Number:
MDL number:
UNSPSC Code:
12352104
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
optical activity
[α]25/D +10°, c = 10 in H2O
optical purity
ee: 95% (GLC)
refractive index
n20/D 1.4548 (lit.)
bp
81 °C/8 mmHg (lit.)
mp
30-32 °C (lit.)
density
0.926 g/mL at 25 °C (lit.)
functional group
amine
hydroxyl
storage temp.
2-8°C
SMILES string
CC(C)[C@H](N)CO
InChI
1S/C5H13NO/c1-4(2)5(6)3-7/h4-5,7H,3,6H2,1-2H3/t5-/m1/s1
InChI key
NWYYWIJOWOLJNR-RXMQYKEDSA-N
Related Categories
Application
(S)-(+)-2-Amino-3-methyl-1-butanol can be used to prepare:
- Imines and oxazolines by reacting with aldehydes and nitriles, respectively.
- Chiral oxazoline derived multidentate ligands containing cyclophosphazene moiety.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
172.4 °F - closed cup
Flash Point(C)
78 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Journal of the Chemical Society. Perkin Transactions 1, 192-192 (1989)
Tetrahedron Letters, 34, 2015-2015 (1993)
Carla Fernandes et al.
Chirality, 29(8), 430-442 (2017-06-14)
Six chiral derivatives of xanthones (CDXs) were covalently bonded to silica, yielding the corresponding xanthonic chiral stationary phases (XCSPs). The new XCSPs were packed into stainless-steel columns with 150 x 4.6 mm i.d. Moreover, the greening of the chromatographic analysis
Enantioselective palladium catalysed allylic substitution with thienyl oxazoline ligands
Frost CG and Williams JMJ
Tetrahedron Letters, 34(12), 2015-2018 (1993)
Asymmetric synthesis of alpha-substituted o-methoxybenzyl alcohols via stereoselective additions to kinetically resolved o-anisaldehyde (tricarbonyl) chromium
Davies SG and Goodfellow CL
Journal of the Chemical Society. Perkin Transactions 1, 1, 192-194 (1989)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service