17354
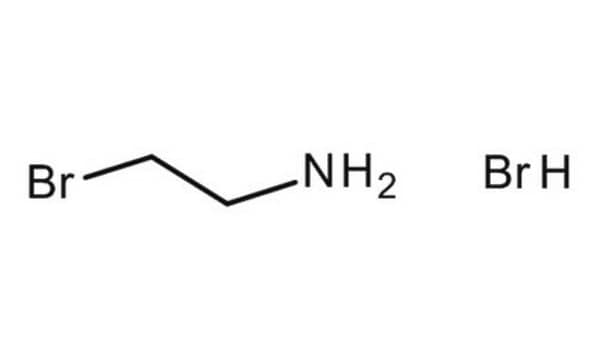
2-(Boc-amino)ethyl bromide
≥97.0% (GC)
Synonym(s):
N-Boc-2-bromoethyl-amine, tert-Butyl N-(2-bromoethyl)carbamate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
BrCH2CH2NHCO2C(CH3)3
CAS Number:
Molecular Weight:
224.10
Beilstein:
2325117
MDL number:
UNSPSC Code:
12352101
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥97.0% (GC)
reaction suitability
reagent type: cross-linking reagent
mp
30-32 °C (lit.)
functional group
Boc
amine
bromo
shipped in
wet ice
storage temp.
−20°C
SMILES string
BrCCNC(OC(C)(C)C)=O
InChI
1S/C7H14BrNO2/c1-7(2,3)11-6(10)9-5-4-8/h4-5H2,1-3H3,(H,9,10)
InChI key
TZRQZPMQUXEZMC-UHFFFAOYSA-N
General description
2-(Boc-amino)ethyl bromide is used in organic synthesis to introduce Boc-protective group and enable the synthesis of various compounds like peptides.
Other Notes
Building block for preparing fluorinated spacers having nucleophilic and electrophilic termini; synthesis of a biphenyl linker
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Convergent Synthesis and Diversity of Amino Acid Based Dendrimers
Arwin J B, et al.
European Journal of Organic Chemistry, 1903-1915 (2001)
L. Chen et al.
Tetrahedron Letters, 39, 3627-3627 (1998)
P. Mougenot et al.
The Journal of Organic Chemistry, 61, 408-408 (1996)
Jinping Cheng et al.
International journal of nanomedicine, 6, 2007-2021 (2011-10-07)
Carbon nanotubes have shown broad potential in biomedical applications, given their unique mechanical, optical, and chemical properties. In this pilot study, carbon nanotubes have been explored as multimodal drug delivery vectors that facilitate antiangiogenic therapy in zebrafish embryos. Three different
Marina Marinović et al.
Molecules (Basel, Switzerland), 25(19) (2020-09-27)
Harmicines represent hybrid compounds composed of β-carboline alkaloid harmine and cinnamic acid derivatives (CADs). In this paper we report the synthesis of amide-type harmicines and the evaluation of their biological activity. N-harmicines 5a-f and O-harmicines 6a-h were prepared by a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service