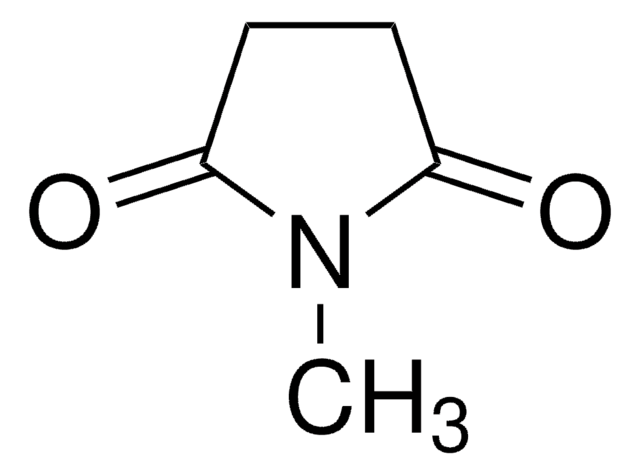
166391
1-Formylpyrrolidine
97%
Synonym(s):
Pyrrolidine-1-carboxaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H9NO
CAS Number:
Molecular Weight:
99.13
Beilstein:
106540
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.479 (lit.)
bp
92-94 °C/15 mmHg (lit.)
density
1.04 g/mL at 25 °C (lit.)
SMILES string
[H]C(=O)N1CCCC1
InChI
1S/C5H9NO/c7-5-6-3-1-2-4-6/h5H,1-4H2
InChI key
AGRIQBHIKABLPJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
1-Formylpyrrolidine is the monomer constituent of gas clathrate inhibitor.
Application
1-Formylpyrrolidine was used in the synthesis of 1-oxa-3,4-dimethyl-5-(1-pyrrolldino)-2,2-di(tert-butyl)silacyclopentane and 1-oxa-4-isopropyl-5-(1-pyrrolidino)-2,2-di(tert-butyl)silacyclopentane.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
203.0 °F - closed cup
Flash Point(C)
95 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Preparation and synthetic utility of oxasilacyclopentane acetals derived from siliranes.
Shaw JT and Woerpel KA.
Tetrahedron, 53(48), 16597-16606 (1997)
Y Tanaka et al.
Journal of medicinal chemistry, 37(13), 2071-2078 (1994-06-24)
New compounds were synthesized by structural modification of 1-[1-(4-phenylbutanoyl)-L-prolyl]-pyrrolidine (SUAM-1221, 1) or 1-[1-(benzyloxycarbonyl)-L-proly]prolinal (Z-Pro-prolinal,2) and were tested for in vitro inhibitory activities against purified prolyl endopeptidase (PEP) from canine brain. In a series of compounds which lack a formyl or
Marco Pallavicini et al.
Bioorganic & medicinal chemistry letters, 14(23), 5827-5830 (2004-10-27)
Homochiral E and Z isomers of N-methylprolinal O-isopropyloxime and (1-methyl-2-pyrrolidinyl)methoxyimines were synthesized as candidate bioisosteres of nicotine and its isoxazolic analogue ABT 418. Two of them, namely (S)-2-isopropylideneaminooxymethyl- and (Z)-(S)-2-ethylideneaminooxymethyl-1-methylpyrrolidine, proved to bind at alpha4beta2 nicotinic acetylcholine receptor with submicromolar
M Nanri et al.
Nihon yakurigaku zasshi. Folia pharmacologica Japonica, 89(6), 323-329 (1987-06-01)
Based on the results of a previous report that prolyl endopeptidase (PPCE) inhibitors facilitated the acquisition of active avoidance response and retarded the extinction of the response, further studies were made on the effect of PPCE inhibitors on learning and
Franck Sobrio et al.
Nuclear medicine and biology, 35(3), 377-385 (2008-03-22)
[11C]-SIB-1553A ((+/-)-4-[2-((N-[11C]-methyl)-2-pyrrolidinyl)ethyl]thiophenol) was labelled with carbon-11 (t1/2=20.4 min) and evaluated in vivo as potential radiotracer for noninvasive assessment of the beta4 subunit nicotinic acetylcholine neurotransmission system with positron emission tomography (PET). The labelling precursor was obtained within five steps from
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service