All Photos(2)
About This Item
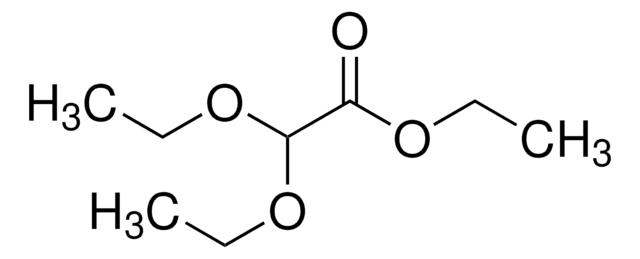
Linear Formula:
CH3COOCH(OCH2CH3)2
CAS Number:
Molecular Weight:
162.18
Beilstein:
1209886
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
form
liquid
refractive index
n20/D 1.399 (lit.)
density
0.993 g/mL at 25 °C (lit.)
functional group
ester
storage temp.
2-8°C
SMILES string
CCOC(OCC)OC(C)=O
InChI
1S/C7H14O4/c1-4-9-7(10-5-2)11-6(3)8/h7H,4-5H2,1-3H3
InChI key
IRUNKQSGDBYUDC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Diethoxymethyl acetate reacts with 5-amino-6-ribitylaminouracil hydrochloride to yield 6-dioxo-(1H,3H)-9-N-ribitylpurine.
Application
Diethoxymethyl acetate was used in the synthesis of D-mannosyl, D-galactosyl and D-glucosyl theophylline nucleosides.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
80.6 °F - closed cup
Flash Point(C)
27 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
M Cushman et al.
Bioorganic & medicinal chemistry, 6(4), 409-415 (1998-05-23)
2,6-Dioxo-(1H,3H)-9-N-ribitylpurine (6) and 2,6-dioxo-(1H,3H)-8-aza-9-N-ribitylpurine (7) have been synthesized and evaluated as inhibitors of lumazine synthase and riboflavin synthase. Reaction of 5-amino-6-ribitylaminouracil hydrochloride (8) with diethoxymethyl acetate (9) afforded the purine 6, while diazotization of 8 afforded the 8-aza purine 7.
Rodrigo Rico-Gómez et al.
Carbohydrate research, 343(5), 855-864 (2008-02-16)
The synthesis of D-mannosyl, D-galactosyl and D-glucosyl theophylline nucleosides by diethoxymethyl acetate (DEMA)-induced cyclization of 4-amino-5-glycosylideneimino-1,3-dimethyluracil is reported. 8-Methyltheophylline derivatives of the same sugars were also prepared by Ac(2)O/H(+)-induced cyclization of their imine precursors. This approach has allowed beta-D-mannopyranosyl-, alpha-D-galactofuranosyl-
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service