15420
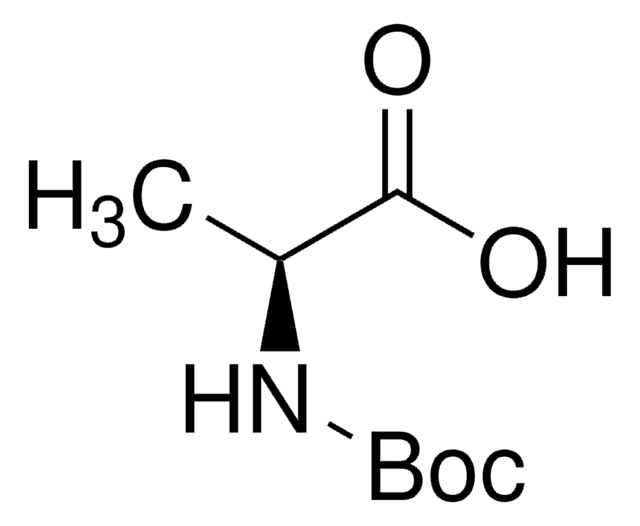
Boc-Gly-OH
≥99.0% (T), for peptide synthesis
Synonym(s):
N-(tert-Butoxycarbonyl)glycine, Boc-glycine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)3COCONHCH2COOH
CAS Number:
Molecular Weight:
175.18
Beilstein:
1101514
EC Number:
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
product name
Boc-Gly-OH, ≥99.0% (T)
Quality Level
Assay
≥99.0% (T)
form
powder or crystals
reaction suitability
reaction type: Boc solid-phase peptide synthesis
ign. residue
≤0.05%
mp
86-89 °C (lit.)
86-89 °C
application(s)
peptide synthesis
SMILES string
CC(C)(C)OC(=O)NCC(O)=O
InChI
1S/C7H13NO4/c1-7(2,3)12-6(11)8-4-5(9)10/h4H2,1-3H3,(H,8,11)(H,9,10)
InChI key
VRPJIFMKZZEXLR-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Boc-Gly-OH can be used:
- For the esterification reaction to synthesize N-Boc amino acid esters for peptide chemistry.
- For the synthesis of tripeptide H-Gly-Pro-Glu-OH, analogs of neuroprotective drugs.
- As a promoter for the allylation of hydrazones and isatin.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Analogues of the neuroprotective tripeptide Gly-Pro-Glu (GPE): synthesis and structure-activity relationships.
De Diego SAA, et al.
Bioorganic & Medicinal Chemistry Letters, 15(9), 2279-2283 (2005)
Ceric ammonium nitrate (CAN) mediated esterification of N-Boc amino acids allows either retention or removal of the N-Boc group.
Kuttan A, et al.
Tetrahedron Letters, 45(12), 2663-2665 (2004)
L Varga et al.
Hepato-gastroenterology, 27(2), 146-149 (1980-04-01)
The catabolism of the 14C-labelled pentagastrin was investigated in rats. The following results were found: -- the labelled BOC-glycine fragment is split off the pentagastrin in the small intestine -- it is absorbed through the intestinal wall, and -- enters
Wen-Ren Li et al.
The Journal of organic chemistry, 67(14), 4702-4706 (2002-07-06)
A new approach to a CD45 protein tyrosine phosphatase inhibitor, pulchellalactam, is described. The key step of the sequence involves addition and elimination of an enolic lactam in a single step and 70% yield, employing an organocuprate reagent. The resulting
Alexey Krushelnitsky et al.
Journal of magnetic resonance (San Diego, Calif. : 1997), 182(2), 339-342 (2006-07-21)
As demonstrated by means of the one-dimensional solid-state MAS exchange experiment (CODEX), the rate of the proton driven spin diffusion between backbone (15)N nuclei in totally enriched protein depends strongly on the magic angle spinning (MAS) frequency: spin diffusion at
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service