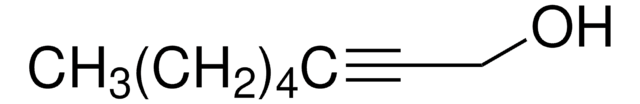127280
1-Octyn-3-ol
96%
Synonym(s):
(RS)-1-Octyn-3-ol, (±)-1-Octyn-3-ol, 1-Ethynyl-1-hexanol, 3-Hydroxyoct-1-yne
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
Quality Level
Assay
96%
form
liquid
refractive index
n20/D 1.441 (lit.)
bp
83 °C/19 mmHg (lit.)
density
0.864 g/mL at 25 °C (lit.)
SMILES string
CCCCCC(O)C#C
InChI
1S/C8H14O/c1-3-5-6-7-8(9)4-2/h2,8-9H,3,5-7H2,1H3
InChI key
VUGRNZHKYVHZSN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
1-Octyn-3-ol is a racemic intermediate formed during the synthesis of enantiomerically pure secondary alcohols with sterically similar substituents.
Application
1-Octyn-3-ol was used in the synthesis of synthetic tricolorin A, a novel tetrasaccharide macrolactone that is a natural herbicide†.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
145.4 °F - closed cup
Flash Point(C)
63 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Daniel P. Larson et al.
The Journal of organic chemistry, 62(24), 8406-8418 (2001-10-24)
Tricolorin A (1) is a novel tetrasaccharide macrolactone that is a natural herbicide. In this paper is reported a total synthesis of 1. Coupling of hydroxy ester 18 with D-fucosyl trichloroacetimidate 23 gave fucoside 24. Removal of the C-2 pivaloyl
José-Luis Abad et al.
The Journal of organic chemistry, 68(13), 5351-5356 (2003-06-21)
A novel chemoenzymatic strategy for the synthesis of enantiomerically pure secondary alcohols with sterically similar substituents is described. The key step is the kinetic lipase-catalyzed resolution of racemic mixtures of substituted propargylic alcohols. The efficiency of this new approach was
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service