125415
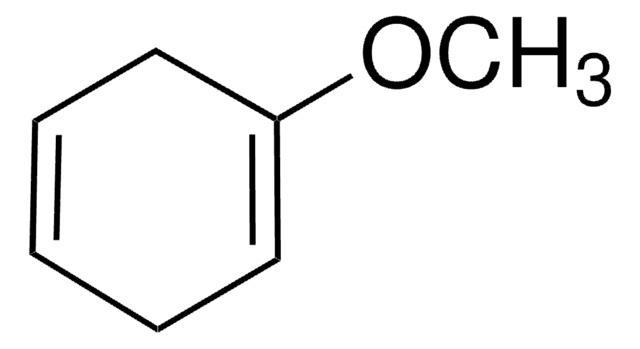
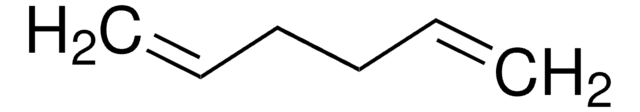
1,4-Cyclohexadiene
97%
Synonym(s):
1,4-Dihydrobenzene
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C6H8
CAS Number:
Molecular Weight:
80.13
Beilstein:
1900733
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
contains
~0.1% hydroquinone as stabilizer
impurities
3% benzene
refractive index
n20/D 1.472 (lit.)
bp
88-89 °C (lit.)
density
0.847 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
C1C=CCC=C1
InChI
1S/C6H8/c1-2-4-6-5-3-1/h1-2,5-6H,3-4H2
InChI key
UVJHQYIOXKWHFD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
1,4-Cyclohexadiene is an effective hydrogen donor for catalytic hydrogenation reactions. It can rapidly replace benzyl groups of N-benzyloxycarbamates, benzyl esters, benzyl ethers and benzyl amines with hydrogen. It forms benzene at elevated temperatures in the presence of a ruthenium(II)-triphenylphosphine catalyst.
Application
1,4-Cyclohexadiene (1,4-CHD) was used to study the formation of parent ion from heavy fragmentation of 1,4-CHD on irradiation with a high-intensity laser pulse.
Useful for the reduction of radical intermediates formed in electron-transfer mediated ring-opening reactions
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Carc. 1A - Flam. Liq. 2 - Muta. 1B - STOT RE 2
Target Organs
Blood
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
19.4 °F - closed cup
Flash Point(C)
-7 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Organometallics, 25, 5456-5456 (2006)
A key factor in parent and fragment ion formation on irradiation with an intense femtosecond laser pulse.
Harada H, et al.
Chemical Physics Letters, 342(5), 563-570 (2001)
Rapid removal of protecting groups from peptides by catalytic transfer hydrogenation with 1, 4-cyclohexadiene.
Felix AM,et al.
The Journal of Organic Chemistry, 43(21), 4194-4196 (1978)
Monika Ali Khan et al.
Chemical communications (Cambridge, England), 47(1), 215-217 (2010-08-24)
A cyclohexadiene ligand prepared by microbial arene 1,2-dihydroxylation undergoes spontaneous rearrangement upon complexation to tricarbonyliron(0). Subsequent iron removal affords a novel route to formal arene 2,3-dihydroxylation products enantiomeric to those obtainable by direct microbial arene oxidation.
Kyung-Bin Cho et al.
Chemical communications (Cambridge, England), 48(16), 2189-2191 (2012-01-19)
DFT calculated barriers for C-H activation of 1,4-cyclohexadiene by nonheme iron(IV)-oxo and iron(III)-superoxo species show that the experimental trends can be explained if the spin inversion probability of the TMC iron(IV)-oxo is assumed to be poor. Also, the TMC iron(III)-superoxo
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service



![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)
![[Ir(dtbbpy)(ppy)2]PF6](/deepweb/assets/sigmaaldrich/product/structures/158/329/2544d673-d267-4aa1-8f46-2652aad4bfa0/640/2544d673-d267-4aa1-8f46-2652aad4bfa0.png)