123617
Benzo[h]quinoline
97%
Synonym(s):
1-Naphthoquinoline, 7,8-Benzoquinoline
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C13H9N
CAS Number:
Molecular Weight:
179.22
Beilstein:
120249
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
bp
338 °C/719 mmHg (lit.)
mp
48-50 °C (lit.)
SMILES string
c1ccc2c(c1)ccc3cccnc23
InChI
1S/C13H9N/c1-2-6-12-10(4-1)7-8-11-5-3-9-14-13(11)12/h1-9H
InChI key
WZJYKHNJTSNBHV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Benzo[h]quinoline was used to study the mutagenic activities of benzo[f]quinoline, benzo[h]quinolone and a number of their derivatives in strain TA 100 of Salmonella typhimurium. It was used in determination of nitrogen-containing polynuclear aromatic hydrocarbons in the gaseous products of the thermal degradation of polymers by HPLC- fluorescence detection. It was used as starting reagent for the synthesis of osmium and ruthenium complexes containing an N-heterocyclic carbene ligand.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
M Wilhelm et al.
Journal of chromatography. A, 878(2), 171-181 (2000-06-24)
A method for the simultaneous determination of 22 nitrogen-containing polynuclear aromatic hydrocarbons (PAHs) (15 azaarenes and seven amino-PAHs) in the gaseous products of the thermal degradation of polymers is described. After desorption and clean-up using cation-exchange chromatography (PRS cartridge) the
E J LaVoie et al.
Japanese journal of cancer research : Gann, 78(2), 139-143 (1987-02-01)
The environmental occurrence and mutagenic activity of quinoline and benzoquinolines are well-documented. In this study, the relative carcinogenic activities of quinoline, benzo[f]quinoline, benzo[h]quinoline, and phenanthridine were evaluated in newborn mice. Mice were injected intraperitoneally on the first, eighth, and fifteenth
E J LaVoie et al.
Carcinogenesis, 4(9), 1133-1138 (1983-09-01)
Benzo[f]quinoline and benzo[h]quinoline are widespread environmental pollutants which have been found to be mutagenic. The metabolism of benzo[f]quinoline and benzo[h]quinoline was investigated using a liver homogenate from Aroclor-pretreated rats. The metabolites of benzo[f]quinoline which were identified were 7,8-dihydroxy-7,8-dihydrobenzo[f]quinoline, 9,10-dihydroxy-9,10-dihydrobenzo[f]quinoline, 7-hydroxybenzo[f]quinoline
S Kumar et al.
Cancer research, 49(1), 20-24 (1989-01-01)
The mutagenic activities of benzo[f]quinoline, benzo[h]quinoline, and a number of their derivatives, including dihydrodiols, K-region oxides, diol epoxides, and tetrahydroepoxides, were assessed in strain TA 100 of Salmonella typhimurium. The dihydrodiol derivatives of benzo[f]quinoline and benzo[h]quinoline were also tested for
Osmium and Ruthenium Complexes Containing an N-Heterocyclic Carbene Ligand Derived from Benzo [h] quinoline.
Esteruelas MA, et al
Organometallics, 26(21), 5239-5245 (2007)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service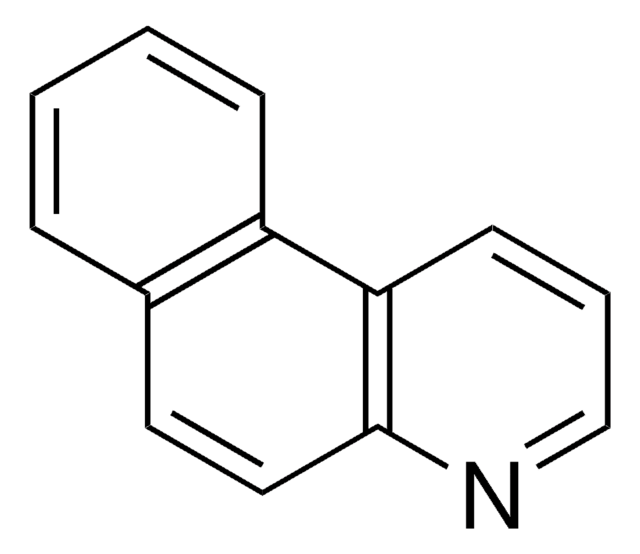










![Dibenz[c,h]acridine BCR®, certified reference material](/deepweb/assets/sigmaaldrich/product/structures/364/643/698df9fb-5b7d-467a-b47e-c8318e2ed298/640/698df9fb-5b7d-467a-b47e-c8318e2ed298.png)
![Benz[g]isoquinoline-5,10-dione 99%](/deepweb/assets/sigmaaldrich/product/structures/484/029/288c4a9d-19c2-4b51-82c1-f43b50ea05b0/640/288c4a9d-19c2-4b51-82c1-f43b50ea05b0.png)