12050
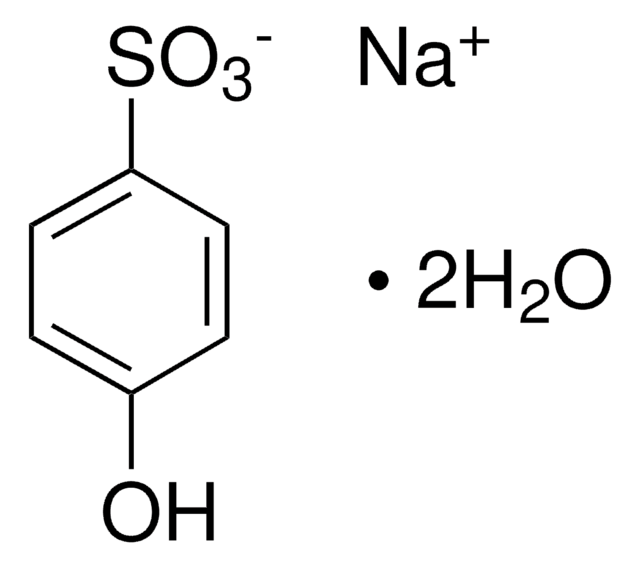
2-Formylbenzenesulfonic acid sodium salt
≥95.0% (T)
Synonym(s):
2-Sulfobenzaldehyde sodium salt, Benzaldehyde-2-sulfonic acid sodium salt, Sodium 2-formyl-benzolsulfonate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C7H5NaO4S
CAS Number:
Molecular Weight:
208.17
Beilstein:
4040884
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥95.0% (T)
form
solid
solubility
H2O: soluble 1 g/10 mL, clear, colorless to faintly brownish-yellow
functional group
aldehyde
sulfonic acid
SMILES string
[Na+].[O-]S(=O)(=O)c1ccccc1C=O
InChI
1S/C7H6O4S.Na/c8-5-6-3-1-2-4-7(6)12(9,10)11;/h1-5H,(H,9,10,11);/q;+1/p-1
InChI key
ADPUQRRLAAPXGT-UHFFFAOYSA-M
General description
2-Formylbenzenesulfonic acid sodium salt reacts with chitosan in the presence of sodium cyanoborohydride to yield N-benzyl derivatives.
Application
2-Formylbenzenesulfonic acid sodium salt was used as precursor to test the ability of fungal strains for transformation of phenolic and non-phenolic precursors into stable and non-toxic dyes.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Hao-Xuan Guo et al.
Polymers, 12(2) (2020-02-08)
In this study, water-soluble, narrow-band-gap polymers containing reactive groups were prepared by the addition-condensation of pyrrole (Pyr), benzaldehyde-2-sulfonic acid sodium salt (BS), and terephthalaldehydic acid (TPA) or p-hydroxybenzaldehyde (p-HB). TPA and p-HB were used for the post-crosslinking reaction between polymers.
NMR characterization of N-benzyl sulfonated derivatives of chitosan.
Crini G, et al.
Carbohydrate Polymers, 33(2), 145-151 (1997)
Jolanta Polak et al.
Microbial cell factories, 9, 51-51 (2010-07-06)
Chemical methods of producing dyes involve extreme temperatures and unsafe toxic compounds. Application of oxidizing enzymes obtained from fungal species, for example laccase, is an alternative to chemical synthesis of dyes. Laccase can be replaced by fungal biomass acting as
Sonja Rittchen et al.
Biochemical pharmacology, 182, 114277-114277 (2020-10-11)
Life-threatening inflammatory conditions such as acute respiratory distress syndrome or sepsis often go hand in hand with severe vascular leakage. During inflammation, endothelial cell integrity and intact barrier function are crucial to limit leukocyte and plasma extravasation. Prostaglandin D2 (PGD2)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service