109606
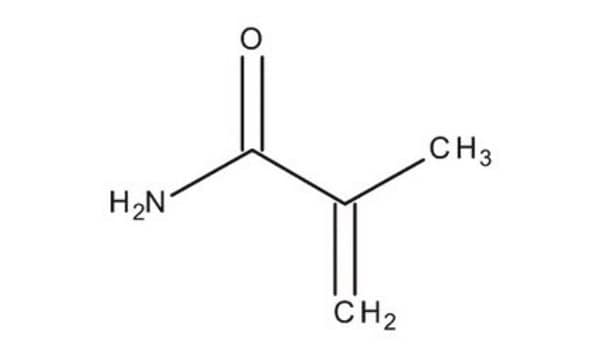
Methacrylamide
98%
Synonym(s):
2-Methylacrylamide, 2-Methylpropenamide, Methacrylic acid amide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
H2C=C(CH3)CONH2
CAS Number:
Molecular Weight:
85.10
Beilstein:
605397
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
98%
form
solid
mp
106-110 °C (lit.)
SMILES string
CC(=C)C(N)=O
InChI
1S/C4H7NO/c1-3(2)4(5)6/h1H2,2H3,(H2,5,6)
InChI key
FQPSGWSUVKBHSU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Methacrylamide is a functional monomer used inthe preparation of thermo-sensitive hydrogels, polymer latexes, and self-etchingprimers with a longer shelf life. It can also be used to prepare dispersionsuspensions to synthesize biocompatible HA/sol-gel glass mixtures.
Application
- Synthesis of non-fouling poly brushes by photoinduced SET-LRP: This study highlights the use of photoinduced SET-LRP for the polymerization of methacrylamide, emphasizing its efficiency and the quality of the resulting polymers for non-fouling applications (M Vorobii, A de los Santos Pereira, 2015).
- Hydrolytic stability of methacrylamide and methacrylate in gelatin methacryloyl: The study investigates the hydrolytic stability of methacrylamide within gelatin methacryloyl, highlighting its stability and potential in biomedical applications (J Zheng, M Zhu, G Ferracci, NJ Cho, 2018).
- Two-step mechanisms of tumor selective delivery of N-(2-hydroxypropyl) methacrylamide copolymer conjugated with pirarubicin via an acid-cleavable linkage: This paper discusses the development of a copolymer conjugate for targeted cancer therapy, showcasing a two-step mechanism for enhanced drug delivery (H Nakamura, T Etrych, P Chytil, M Ohkubo, 2014).
- Backbone Degradable N-(2-Hydroxypropyl)methacrylamide Copolymer Conjugates with Gemcitabine and Paclitaxel: The research focuses on degradable copolymer conjugates for delivering cancer therapeutics, noting significant effects on tumor reduction and highlighting the impact of molecular weight (J Yang, R Zhang, H Pan, Y Li, Y Fang, 2017).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - STOT RE 2 - STOT SE 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Patrik Sobolčiak et al.
Macromolecular rapid communications, 34(8), 635-639 (2013-02-13)
A novel cationic polymer poly(N,N-dimethyl-N-[3-(methacroylamino) propyl]-N-[2-[(2-nitrophenyl)methoxy]-2-oxo-ethyl]ammonium chloride) is synthesized by free-radical polymerization of N-[3-(dimethylamino)propyl] methacrylamide and subsequent quaternization with o-nitrobenzyl 2-chloroacetate. The photolabile o-nitrobenzyl carboxymethyl pendant moiety is transformed to the zwitterionic carboxybetaine form upon the irradiation at 365 nm.
Cesar Rodriguez-Emmenegger et al.
Macromolecular rapid communications, 32(13), 958-965 (2011-06-08)
Among the class of zwitterionic polymers poly(carboxybetaine)s (poly(CB)s) are unique, emerging as the only ultra-low fouling materials known allowing the preparation of biosensors, fouling resistant nanoparticles, and non-adhesive surfaces for bacteria. Poly(carboxybetaine methacrylate) and poly(carboxybetaine acrylamide) have been prepared via
Jian-Lian Chen et al.
Electrophoresis, 32(3-4), 398-407 (2011-02-08)
Nanoparticles exhibiting favorable surface-to-volume ratios create efficient stationary phases for electrochromatography. New nanomaterials derived from chitosan (CS) were immobilized onto modified capillaries for use as the chiral stationary phase (CSP) in open-tubular electrochromatography. This immobilization was achieved through the copolymerization
Ellen Verheyen et al.
Journal of controlled release : official journal of the Controlled Release Society, 156(3), 329-336 (2011-09-14)
We report an efficient strategy to conjugate methacrylamide moieties to the lysine units of lysozyme for co-polymerization and subsequent triggered release from hydrogels. Two novel linker molecules, containing an ester bond and/or a disulfide bond for temporary immobilization, were synthesized
Fluorinated methacrylamide chitosan hydrogel systems as adaptable oxygen carriers for wound healing.
Asanka Wijekoon et al.
Acta biomaterialia, 9(3), 5653-5664 (2012-11-07)
In this study a series of novel, biocompatible hydrogels able to repeatedly takeup and deliver oxygen at beneficial levels have been developed by conjugating various perfluorocarbon (PFC) chains to methacrylamide chitosan via Schiff base nucleophilic substitution, followed by photopolymerization to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service