SML1896
VH298
≥98% (HPLC)
Synonym(s):
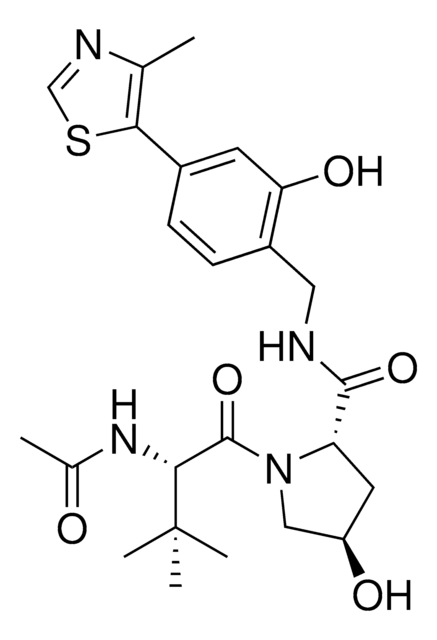
(2S,4R)-1-((S)-2-(1-Cyanocyclopropanecarboxamido)-3,3-dimethylbutanoyl)-4-hydroxy-N-(4-(4-methylthiazol-5-yl)benzyl)pyrrolidine-2-carboxamide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C27H33N5O4S
CAS Number:
Molecular Weight:
523.65
UNSPSC Code:
12352200
NACRES:
NA.77
Recommended Products
ligand
VH298
Quality Level
Assay
≥98% (HPLC)
form
powder
reaction suitability
reagent type: ligand
color
white to beige
solubility
DMSO: 10 mg/mL, clear
storage temp.
−20°C
Related Categories
Biochem/physiol Actions
VH298 is a cell-permeable small molecule that disrupts the interaction of VHL with Hypoxia-inducible factor HIF-α, stabilizing HIF-α and inducing a hypoxic response. Hypoxia-inducible factors (HIFs) are oxygen-sensitive transcription factors that regulate hypoxic signalling. They are regulated by oxygen-dependent prolyl hydroxylase domain (PHD) enzymes and polyubiquitination by the von Hippel–Lindau (VHL) Cullin RING E3 ubiquitin ligase complex. Unlike many PHD inhibitors, which have off-target effects, VH298 acts downstream of the PHD enzymes, inhibiting the VHL:HIF-α interaction. VH298 had a Kd of 90 nM. VH298 was shown to induce HIF transcriptional activity, to increase accumulation of HIF-α in HeLa cells and to stimulate erythropoietin production in RCC4 cells.
related product
Product No.
Description
Pricing
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Julianty Frost et al.
Nature communications, 7, 13312-13312 (2016-11-05)
Chemical strategies to using small molecules to stimulate hypoxia inducible factors (HIFs) activity and trigger a hypoxic response under normoxic conditions, such as iron chelators and inhibitors of prolyl hydroxylase domain (PHD) enzymes, have broad-spectrum activities and off-target effects. Here
Olena S Tokareva et al.
Nature communications, 14(1), 6992-6992 (2023-11-02)
Molecules that induce novel interactions between proteins hold great promise for the study of biological systems and the development of therapeutics, but their discovery has been limited by the complexities of rationally designing interactions between three components, and because known
Lauren M Meyers et al.
Toxicology and applied pharmacology, 445, 116041-116041 (2022-05-04)
Transcription factors HIF1 and HIF2 are central regulators of physiological responses to hypoxia and important for normal functioning of tissue stem cells and maintenance of healthy microvasculature. Even modest decreases in HIF activity exert detrimental effects in tissues although it
Yong Hwan Kim et al.
Molecules (Basel, Switzerland), 29(2) (2024-01-23)
Autophagy is a pivotal biological process responsible for maintaining the homeostasis of intracellular organelles. Yet the molecular intricacies of peroxisomal autophagy (pexophagy) remain largely elusive. From a ubiquitin-related chemical library for screening, we identified several inhibitors of the Von Hippel-Lindau
Misty Shuo Zhang et al.
Nature communications, 13(1), 954-954 (2022-02-19)
Hepatocellular carcinoma (HCC) invariably exhibits inadequate O2 (hypoxia) and nutrient supply. Hypoxia-inducible factor (HIF) mediates cascades of molecular events that enable cancer cells to adapt and propagate. Macropinocytosis is an endocytic process initiated by membrane ruffling, causing the engulfment of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service