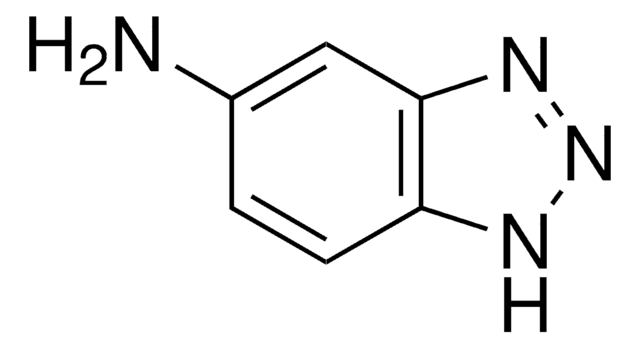
B11400
Benzotriazole
ReagentPlus®, 99%
Synonym(s):
1,2,3-Benzotriazole, 1H-Benzotriazole
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Empirical Formula (Hill Notation):
C6H5N3
CAS Number:
Molecular Weight:
119.12
Beilstein:
112133
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.21
Recommended Products
vapor density
4.1 (vs air)
Quality Level
vapor pressure
0.04 mmHg ( 20 °C)
product line
ReagentPlus®
Assay
99%
form
powder
mp
97-99 °C (lit.)
SMILES string
c1ccc2[nH]nnc2c1
InChI
1S/C6H5N3/c1-2-4-6-5(3-1)7-9-8-6/h1-4H,(H,7,8,9)
InChI key
QRUDEWIWKLJBPS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Benzotriazole is used as a synthetic auxiliary for the preparation of organic derivatives and as a corrosion inhibitors for Cu and its alloys.
Application
Benzotriazole can be used as a reactant to synthesize:
- β-Aminocarbonyl compounds via Mannich reaction of secondary amines and aldehydes in the presence of p-toluenesulfonic acid as a catalyst.
- Acylbenzotriazoles via thionyl chloride catalyzed reaction with nitrobenzoic acids.
- 1-(2-Pyridyl)benzotriazole by reacting with 2-bromopyridine in the presence of toluene as a solvent.
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 2 - Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
338.0 °F - closed cup
Flash Point(C)
170 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Martin Krug et al.
ChemMedChem, 6(1), 63-72 (2010-12-09)
Within the last decade, interest in the development of new anticancer drugs increased mainly from emerging resistance against established drugs, which were found to be limited by the multidrug resistance (MDR) phenomenon. Several anticancer targets have been investigated for the
Synthesis, Characterization and Energetic Properties of 1, 3, 4-Oxadiazoles
Wang Z, et al.
European Journal of Organic Chemistry, 2015, 5183-5188 (2015)
Derivatization of 1-phenyl substituted 4-amino-2-benzazepin-3-ones: evaluation of Pd-catalyzed coupling reactions
Ballet S, et al.
Tetrahedron, 63, 3718-3727 (2007)
Inhibition of copper corrosion by 1, 2, 3-benzotriazole: a review
Matjavz F et al.
Corrosion Science, 52, 2737-2749 (2010)
Benzotriazole as a synthetic auxiliary: benzotriazolylalkylations and benzotriazole-mediated heteroalkylation
Alan K R et al.
Synthesis, 1994, 445-456 (1994)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service