All Photos(1)
About This Item
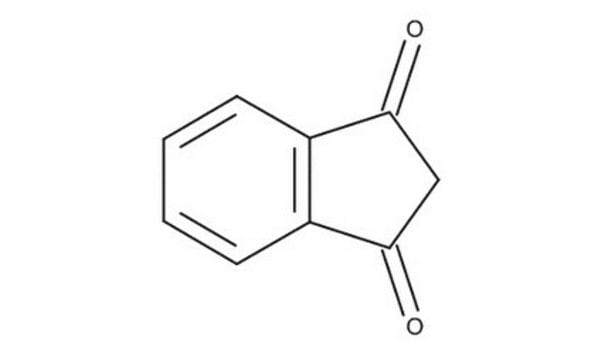
Empirical Formula (Hill Notation):
C9H6O2
CAS Number:
Molecular Weight:
146.14
Beilstein:
1210061
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
129-132 °C (lit.)
SMILES string
O=C1CC(=O)c2ccccc12
InChI
1S/C9H6O2/c10-8-5-9(11)7-4-2-1-3-6(7)8/h1-4H,5H2
InChI key
UHKAJLSKXBADFT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
V Violet Dhayabaran et al.
Journal of fluorescence, 27(1), 135-150 (2016-10-04)
Novel bioactive complexes of Co(II), Cu(II), Ni(II) and Zn(II) metal ions with Schiff base ligand derived from histidine and 1,3-indandione were synthesized and thoroughly characterized by various analytical and spectral techniques. The biological investigations were carried out to examine the
Halise Inci Gul et al.
Anti-cancer agents in medicinal chemistry, 18(12), 1770-1778 (2018-05-26)
In this study, new azafluorenones, 4-(4-fluorophenyl)-2-(4-substitutedphenyl)-5Hindeno[ 1,2-b] pyridin-5-one, I1-I8 were synthesized and chemical structures were elucidated by spectral analysis. All compounds were reported for the first time here. Compounds were tested in terms of cytotoxicity. They were found as cytotoxins/anticancer
M V Diurno et al.
Bollettino della Societa italiana di biologia sperimentale, 60(1), 79-84 (1984-01-30)
In order to obtain biologically active compounds with a probable anticoagulant activity, some new products, by Michael method from indandione and 2-phenylindandione with ethyl alpha-cyano-beta-aryl-acrylates, have been prepared. The reaction of phenylindandione with methylvinylketone has been also carried out. The
[Effect of S-oxidation on the anticoagulant effects of 4-hydroxycoumarins, 4-hydroxy-2-pyrones and 1,3-indanediones].
K Rehse et al.
Archiv der Pharmazie, 317(3), 262-267 (1984-03-01)
Bilquees Bano et al.
Bioorganic chemistry, 81, 658-671 (2018-09-27)
Current study deals with the evaluation of indane-1,3-dione based compounds as new class of urease inhibitors. For that purpose, benzylidine indane-1,3-diones (1-30) were synthesized and fully characterized by different spectroscopic techniques including EI-MS, HREI-MS, 1H, and 13C NMR. All synthetic
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service