721352
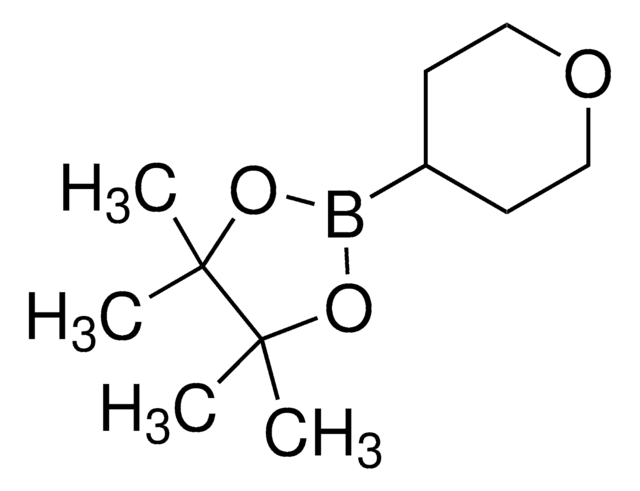
3,6-Dihydro-2H-pyran-4-boronic acid pinacol ester
97%
Synonym(s):
2-(3,6-Dihydro-2H-pyran-4-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C11H19BO3
CAS Number:
Molecular Weight:
210.08
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
63-67 °C
storage temp.
2-8°C
SMILES string
CC1(C)OB(OC1(C)C)C2=CCOCC2
InChI
1S/C11H19BO3/c1-10(2)11(3,4)15-12(14-10)9-5-7-13-8-6-9/h5H,6-8H2,1-4H3
InChI key
DOSGEBYQRMBTGS-UHFFFAOYSA-N
Application
3,6-Dihydro-2H-pyran-4-boronic acid pinacol ester can be used:
- To prepare 1,2-dihydro-2-oxopyridine based endocannabinoid system (ECS) modulators.
- As an intermediate in the synthesis of embryonic ectoderm development (EED) inhibitors.
- To prepare pyrrolotriazine based IRAK4 inhibitors.
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
EEDi-5285: An Exceptionally Potent, Efficacious, and Orally Active Small-Molecule Inhibitor of Embryonic Ectoderm Development
Rej RK, et al.
Journal of medicinal chemistry, 63(13), 7252-7267 (2020)
Modification on the 1, 2-dihydro-2-oxo-pyridine-3-carboxamide core to obtain multi-target modulators of endocannabinoid system
Gado F, et al.
Bioorganic Chemistry, 94, 103353-103353 (2020)
Optimization of permeability in a series of pyrrolotriazine inhibitors of IRAK4
Degorce SL, et al.
Bioorganic & Medicinal Chemistry, 26(4), 913-924 (2018)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)