All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H6ClNO
CAS Number:
Molecular Weight:
179.60
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
Assay
98%
mp
213-216 °C (lit.)
SMILES string
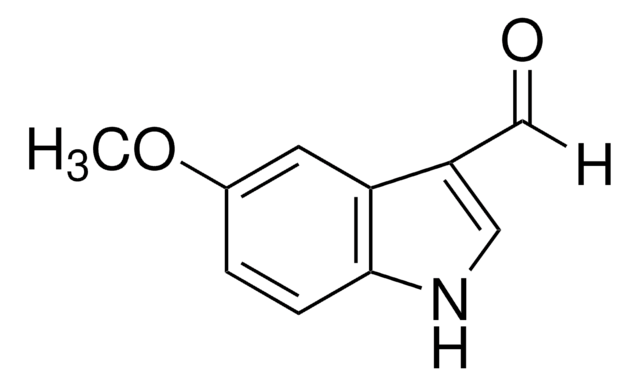
Clc1ccc2[nH]cc(C=O)c2c1
InChI
1S/C9H6ClNO/c10-7-1-2-9-8(3-7)6(5-12)4-11-9/h1-5,11H
InChI key
YXEXOIGXNYITQH-UHFFFAOYSA-N
General description
5-Chloroindole-3-carboxaldehyde, also known as 5-chloro-1H-indole-3-carboxaldehyde, is an indole derivative.
Application
5-Chloroindole-3-carboxaldehyde (5-Chloro-1H-indole-3-carboxaldehyde) may be used in the preparation of:
It may also be used in the preparation of the following hydrazone derivatives:
- 5-chloroindole-3-carboxaldehyde isonicotinoyl hydrazine
- 2′-[(5-chloro-1H-indol-3-yl)methylene]-2-(1H-indol-3-yl)acetohydrazide
- 5-chloro-3-(2,2-dibromovinyl)-1-(2-trimethylsilylethoxymethyl)indole
It may also be used in the preparation of the following hydrazone derivatives:
- 5-chloroindole-3-carboxaldehyde 3-chlorobenzoylhydrazone
- 5-chloroindole-3-carboxaldehyde 4-nitrobenzoylhydrazone
- 5-chloroindole-3-carboxaldehyde 3-methylbenzoylhydrazone
- 5-chloroindole-3-carboxaldehyde 4-methylbenzoylhydrazone
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Kamaleddin Haj Mohammad Ebrahim Tehrani et al.
Iranian journal of pharmaceutical research : IJPR, 14(4), 1077-1086 (2015-12-15)
A series of indole-based aryl(aroyl)hydrazone analogs of antiplatelet indole-3-carboxaldehyde phenylhydrazone were synthesized by the Schiff base formation reaction and their antiplatelet activity was assessed using human platelet rich plasma. The platelet concentrate was obtained using a two-step centrifugation protocol and
Tandem Suzuki-Miyaura cross-coupling/dehydrobromination of 1, 1-dibromoalkenes to alkynes with a cyclobutene-1, 2-diylbis (imidazolium) salt as catalyst precursor.
Rahimi A and Schmidt A.
Synthesis, 2010(15), 2621-2625 (2010)
2?-[(5-Chloro-1H-indol-3-yl) methylene]-2-(1H-indol-3-yl) acetohydrazide.
Ali HM, et al.
Acta Crystallographica Section E, Structure Reports Online, 63(4), o1807-o1808 (2007)
Electrochemical behavior of indole-3-carboxaldehyde izonicotinoyl hydrazones: discussion on possible biological behavior
Shirinzadeh H, et al.
Combinatorial Chemistry & High Throughput Screening, 13(7), 619-627 (2010)
Ming-Zhi Zhang et al.
European journal of medicinal chemistry, 92, 776-783 (2015-01-31)
Streptochlorin, first isolated as a new antibiotic in 1988 from the lipophilic extracts of the mycelium of a Streptomyces sp, is an indole natural products with a variety of biological activities. Based on the methods developed for the synthesis of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service