520160
Shi Epoxidation Diketal Catalyst
98%
Synonym(s):
1,2:4,5-Di-O-isopropylidene-β-D-erythro-2,3-hexodiulo-2,6-pyranose
About This Item
Recommended Products
Assay
98%
optical activity
[α]20/D −120.9°, c = 1 in chloroform
greener alternative product characteristics
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
102-104 °C (lit.)
greener alternative category
, Aligned
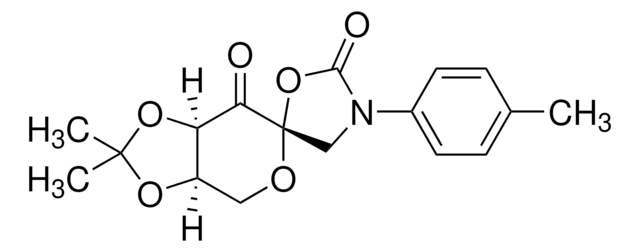
SMILES string
CC1(C)O[C@@H]2CO[C@]3(COC(C)(C)O3)C(=O)[C@@H]2O1
InChI
1S/C12H18O6/c1-10(2)15-6-12(18-10)9(13)8-7(5-14-12)16-11(3,4)17-8/h7-8H,5-6H2,1-4H3/t7-,8-,12+/m1/s1
InChI key
IVWWFWFVSWOTLP-RWYTXXIDSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
An Efficient Catalytic Asymmetric Epoxidation Method
Use of an Iridium-Catalyzed Redox-Neutral Alcohol-Amine Coupling on Kilogram Scale for the Synthesis of a GlyT1 Inhibitor
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Catalytic asymmetric epoxidation of alkenes has been the focus of many research efforts over the past two decades, the best known methods probably being those developed by Sharpless and Jacobsen-Katsuki.
Catalytic asymmetric epoxidation of alkenes has been the focus of many research efforts over the past two decades, the best known methods probably being those developed by Sharpless and Jacobsen-Katsuki.
Catalytic asymmetric epoxidation of alkenes has been the focus of many research efforts over the past two decades, the best known methods probably being those developed by Sharpless and Jacobsen-Katsuki.
Catalytic asymmetric epoxidation of alkenes has been the focus of many research efforts over the past two decades, the best known methods probably being those developed by Sharpless and Jacobsen-Katsuki.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service