26235
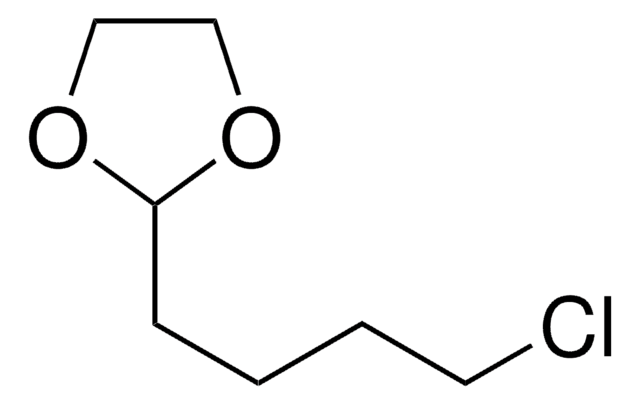
2-(3-Chloropropyl)-1,3-dioxolane
≥97.0% (GC)
Synonym(s):
4-Chlorobutyraldehyde ethylene acetal
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H11ClO2
CAS Number:
Molecular Weight:
150.60
Beilstein:
1236588
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥97.0% (GC)
form
liquid
refractive index
n20/D 1.453
bp
93-94 °C/12 mmHg (lit.)
density
1.142 g/mL at 20 °C (lit.)
functional group
chloro
ether
SMILES string
ClCCCC1OCCO1
InChI
1S/C6H11ClO2/c7-3-1-2-6-8-4-5-9-6/h6H,1-5H2
InChI key
ZBPUNVFDQXYNDY-UHFFFAOYSA-N
Related Categories
Application
2-(3-Chloropropyl)-1,3-dioxolane (2-(3′-chloropropyl)-1,3-dioxolane) is a masked γ-chlorobutyraldehyde and was used for the introduction of 3-(1,3-dioxolan-2-yl)propyl moiety. It was also used in the synthesis of:
- (±)-histrionicotoxin and (±)-histrionicotoxin 235A using a two-directional strategy
- 4-iodobutyraldehyde, 5-iodovaleraldehyde and 5-iodo-2-petanone
- corresponding phosphonate
Other Notes
Masked γ-chlorobutyraldehyde, useful for the introduction of the 3-(1,3-dioxolan-2-yl)propyl moiety; Preparation and use of the corresponding phosphonate
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
174.2 °F - closed cup
Flash Point(C)
79 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
C.P. Forbes et al.
Journal of the Chemical Society. Perkin Transactions 1, 2353-2353 (1977)
R.E. Abbott et al.
The Journal of Organic Chemistry, 45, 5398-5398 (1980)
Two-directional synthesis. Part 1: A short formal synthesis of (?)-histrionicotoxin and (?)-histrionicotoxin 235A.
Stockman RA.
Tetrahedron Letters, 41(47), 9163-9165 (2000)
S.A. Bal et al.
The Journal of Organic Chemistry, 47, 5045-5045 (1982)
A Nagy et al.
Proceedings of the National Academy of Sciences of the United States of America, 93(6), 2464-2469 (1996-03-19)
A convenient, high yield conversion of doxorubicin to 3'-deamino-3'-(2''-pyrroline-1''-yl)doxorubicin is described. This daunosamine-modified analog of doxorubicin is 500-1000 times more active in vitro than doxorubicin. The conversion is effected by using a 30-fold excess of 4-iodobutyraldehyde in anhydrous dimethylformamide. The
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service