R5653
Monoclonal Anti-hnRNP-Q antibody produced in mouse
clone 18E4, purified immunoglobulin, buffered aqueous solution
Sinónimos:
Anti-Heterogeneous Nuclear Ribonucleoprotein-Q
About This Item
Productos recomendados
origen biológico
mouse
Nivel de calidad
conjugado
unconjugated
forma del anticuerpo
purified immunoglobulin
tipo de anticuerpo
primary antibodies
clon
18E4, monoclonal
Formulario
buffered aqueous solution
mol peso
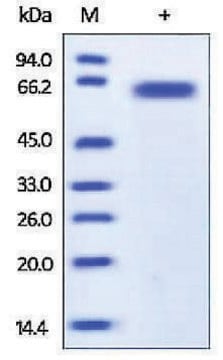
antigen 55-70 kDa
reactividad de especies
mouse, bovine, human, rat, canine, Xenopus, chicken
técnicas
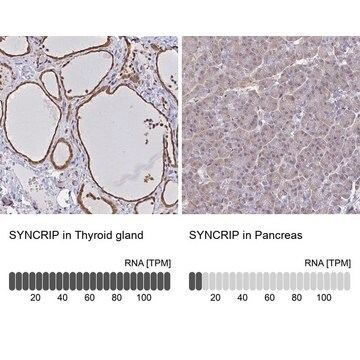
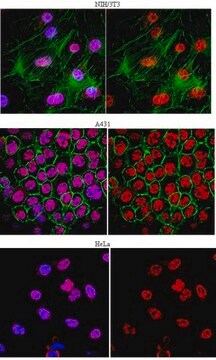
immunohistochemistry: suitable
immunoprecipitation (IP): suitable
indirect ELISA: suitable
microarray: suitable
western blot: 1-2 μg/mL using HeLa total cell extract
isotipo
IgG1
Condiciones de envío
dry ice
temp. de almacenamiento
−20°C
modificación del objetivo postraduccional
unmodified
Información sobre el gen
human ... SYNCRIP(10492)
Descripción general
Especificidad
Inmunógeno
Aplicación
Acciones bioquímicas o fisiológicas
Forma física
Cláusula de descargo de responsabilidad
¿No encuentra el producto adecuado?
Pruebe nuestro Herramienta de selección de productos.
Código de clase de almacenamiento
12 - Non Combustible Liquids
Clase de riesgo para el agua (WGK)
nwg
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico

![Tetrazolo[1,5-a]pyridine-7-carboxylic acid AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/201/135/ec8cce15-d496-44e3-87bd-6c9d39c85482/640/ec8cce15-d496-44e3-87bd-6c9d39c85482.png)



