H7002
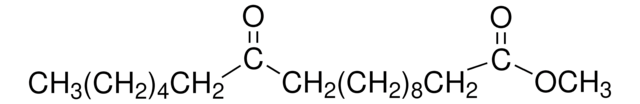
Methyl 12-hydroxystearate
≥99% (GC)
Sinónimos:
12-Hydroxystearic acid methyl ester, Methyl 12-hydroxyoctadecanoate
About This Item
Productos recomendados
Quality Level
assay
≥99% (GC)
form
powder
functional group
ester
lipid type
saturated FAs
shipped in
ambient
storage temp.
−20°C
SMILES string
CCCCCCC(O)CCCCCCCCCCC(=O)OC
InChI
1S/C19H38O3/c1-3-4-5-12-15-18(20)16-13-10-8-6-7-9-11-14-17-19(21)22-2/h18,20H,3-17H2,1-2H3
InChI key
RVWOWEQKPMPWMQ-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Application
- Chemical Changes of Hydroperoxy-, Epoxy-, Keto- and Hydroxy-Model Lipids under Simulated Gastric Conditions.: This study explores the stability and chemical transformations of hydroxy fatty acids, including Methyl 12-hydroxystearate, under digestive conditions, providing insight into dietary fat metabolism and its implications for nutritional sciences (Marquez-Ruiz et al., 2021).
- Stimulation of nitrogen removal in the rhizosphere of aquatic duckweed by root exudate components.: This research highlights the potential environmental applications of Methyl 12-hydroxystearate, as a standard, in enhancing nitrogen cycling, important for studies on wastewater treatment and ecosystem management (Lu et al., 2014).
- Synthesis and evaluation of antioxidant and antifungal activities of novel ricinoleate-based lipoconjugates of phenolic acids.: This study investigates the synthesis of derivatives of Methyl 12-hydroxystearate for potential use in food preservation and pharmaceutical applications, emphasizing its antioxidant and antifungal properties (Reddy et al., 2012).
Biochem/physiol Actions
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico