81838
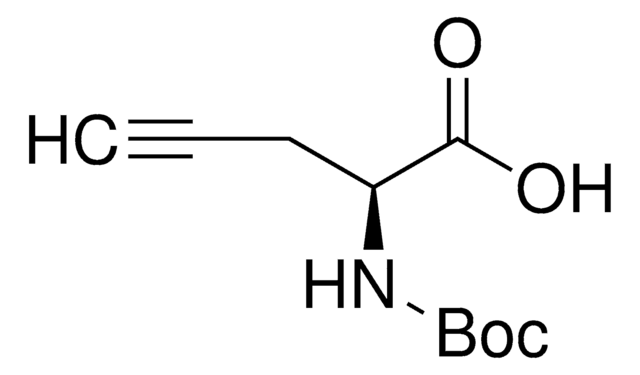
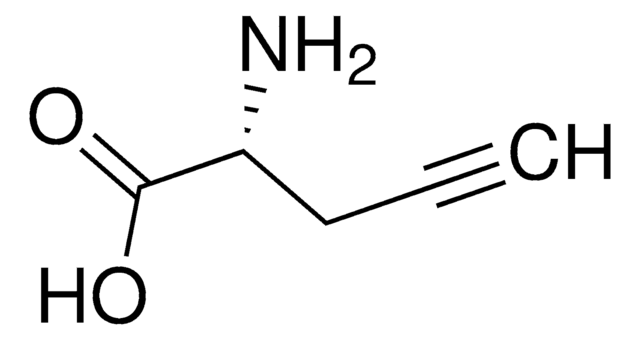
L-C-Propargylglycine
≥99.0% (TLC)
Sinónimos:
L-Propargylglycine, (S)-2-Amino-4-pentynoic acid
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C5H7NO2
Número de CAS:
Peso molecular:
113.11
Beilstein:
2347861
Número MDL:
Código UNSPSC:
12352209
eCl@ss:
32160406
ID de la sustancia en PubChem:
NACRES:
NA.26
Productos recomendados
Nombre del producto
L-C-Propargylglycine, ≥99.0% (TLC)
Nivel de calidad
Ensayo
≥99.0% (TLC)
Formulario
powder
color
white
mp
235-239 °C
aplicaciones
peptide synthesis
temp. de almacenamiento
2-8°C
cadena SMILES
N[C@@H](CC#C)C(O)=O
InChI
1S/C5H7NO2/c1-2-3-4(6)5(7)8/h1,4H,3,6H2,(H,7,8)/t4-/m0/s1
Clave InChI
DGYHPLMPMRKMPD-BYPYZUCNSA-N
Aplicación
Reagent for the irreversible inactivation of γ-cystathionase; affinity labeling reagent for γ-cystathionase and other enzymes; peptides containing this amino acid can be tritiated to high specific radioactivity
Acciones bioquímicas o fisiológicas
L-C-Propargylglycine, a specific inhibitor of H(2)S synthase of cystathionine-γ-lyase (CSE), may be used to study the role of H2S in regulation of biological processes.
L-propargylglycine (PAG), an inhibitor of cystathionine γ-lyase (CSE), is useful in studies of hydrogen sulphide synthesis and bioactivity.
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Bridget Fox et al.
Journal of cellular and molecular medicine, 16(4), 896-910 (2011-06-18)
Hydrogen sulfide (H(2)S) has recently been proposed as an endogenous mediator of inflammation and is present in human synovial fluid. This study determined whether primary human articular chondrocytes (HACs) and mesenchymal progenitor cells (MPCs) could synthesize H(2)S in response to
Emanuela Pupo et al.
Free radical biology & medicine, 51(9), 1765-1773 (2011-08-31)
Hydrogen sulfide (H(2)S) is a gasotransmitter that plays several roles in various tissues, including the cardiovascular system. Because it has been recently proposed to act as a mediator of angiogenesis progression, here we investigate the effects of H(2)S in a
V Gil et al.
British journal of pharmacology, 164(2b), 485-498 (2011-04-14)
The role of hydrogen sulphide (H₂S) as a putative endogenous signalling molecule in the gastrointestinal tract has not yet been established. We investigated the effect of D,L-propargylglycine (PAG), an inhibitor of cystathionine γ-lyase (CSE), amino-oxyacetic acid (AOAA) and hydroxylamine (HA)
Yan Gao et al.
International journal of cardiology, 152(2), 177-183 (2011-02-15)
Hydrogen sulfide (H(2)S) displays anti-inflammatory and cytoprotective activities to attenuate myocardial ischemia-reperfusion (MIR)-induced injury, but its role in MIR in diabetics is not known. This study was undertaken to investigate whether H(2)S plays a protective role in MIR in diabetic
Metabolic consequences of affinity labeling of cystathionase and alanine aminotransferase by L-propargylglycine in vivo.
S Shinozuka et al.
European journal of biochemistry, 124(2), 377-382 (1982-05-17)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico