70050
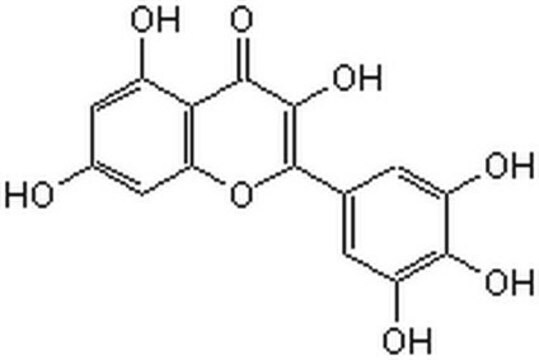
Myricetin
≥96.0% (HPLC)
Sinónimos:
3,3′,4′,5,5′,7-Hexahydroxyflavone, Cannabiscetin, Myricetol
About This Item
Productos recomendados
Quality Level
assay
≥96.0% (HPLC)
form
powder
mp
≥300 °C
>300 °C (lit.)
solubility
ethanol: 10 mg/mL, clear to very faintly turbid, yellow to very deep greenish-yellow
application(s)
metabolomics
vitamins, nutraceuticals, and natural products
SMILES string
Oc1cc(O)c2C(=O)C(O)=C(Oc2c1)c3cc(O)c(O)c(O)c3
InChI
1S/C15H10O8/c16-6-3-7(17)11-10(4-6)23-15(14(22)13(11)21)5-1-8(18)12(20)9(19)2-5/h1-4,16-20,22H
InChI key
IKMDFBPHZNJCSN-UHFFFAOYSA-N
Gene Information
human ... CYP1A2(1544)
mouse ... Hexa(15211)
rat ... Il4(287287) , Tnf(24835)
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Application
- to study its preventive effect as an antioxidant on noise-induced hearing loss (NIHL) in rats
- as a flavonoid compound to test antiviral activity of Bourbon virus (BRBV) and in inhibition of RNA-dependent RNA polymerase (RdRP)
- to study its effect as a treatment on biofilms of Streptococcus mutans and Candida albicans
- as a reference standard for the quantification of phenolic compounds from Juniperus species
Biochem/physiol Actions
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Antioxidants protect biological systems from oxidative damage produced by oxygen-containing free radicals and from redoxactive transition metal ions such as iron, copper, and cadmium.
Protocolos
Protocol for HPLC Analysis of Flavonoids on Ascentis® RP-Amide
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico