P35405
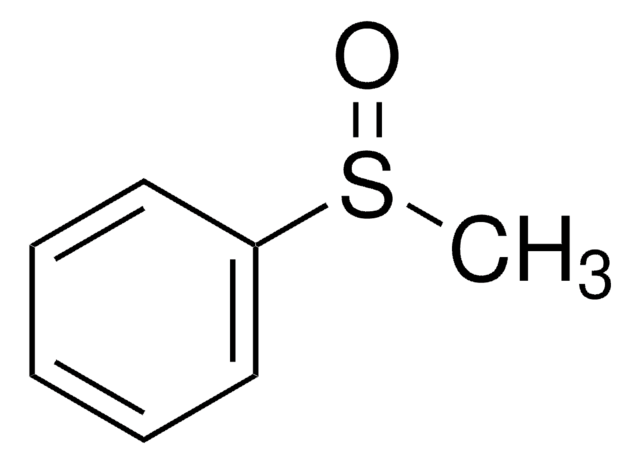
Diphenyl sulfoxide
96%
Sinónimos:
Phenyl sulfoxide
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
(C6H5)2SO
Número de CAS:
Peso molecular:
202.27
Beilstein:
1908444
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de la sustancia en PubChem:
NACRES:
NA.22
Productos recomendados
Nivel de calidad
Ensayo
96%
Formulario
crystals
bp
206-208 °C/13 mmHg (lit.)
mp
69-71 °C (lit.)
cadena SMILES
O=S(c1ccccc1)c2ccccc2
InChI
1S/C12H10OS/c13-14(11-7-3-1-4-8-11)12-9-5-2-6-10-12/h1-10H
Clave InChI
JJHHIJFTHRNPIK-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Aplicación
- Preparation of radiochemicals: Diphenyl sulfoxide plays a role in the synthesis of [(11)C]cyanide from [(11)C]methyl iodide, facilitating rapid and efficient production of radiochemicals for medical imaging applications (Kikuchi et al., 2022).
- Catalytic oxidation processes: The photocatalytic and catalytic oxidation of diphenyl sulphide to sulfoxide and sulfone was examined, highlighting the effectiveness of hydrogen peroxide and TiO2 polymorphs in optimizing chemical processes (Mikrut et al., 2022).
- Dielectric properties research: The study on dielectric properties of high organic sulfur coal highlighted the modeling of sulfur compounds, which could include diphenyl sulfoxide, enhancing our understanding of materials science in energy sectors (Cai et al., 2019).
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
S Yoshihara et al.
Drug metabolism and disposition: the biological fate of chemicals, 18(6), 876-881 (1990-11-01)
To evaluate the metabolic capacity of intact guinea pig liver under normoxic and hypoxic conditions, oxidative and reductive metabolism of diphenyl sulfoxide (DPSO) was studied by the nonrecirculating perfusion method in situ. DPSO was exclusively converted into diphenyl sulfone (DPSO2)
Martin A Fascione et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 18(10), 2987-2997 (2012-02-02)
Sulfoxides are frequently used in organic synthesis as chiral auxiliaries and reagents to mediate a wide variety of chemical transformations. For example, diphenyl sulfoxide and triflic anhydride can be used to activate a wide range of glycosyl donors including hemiacetals
J A Kozlowski et al.
Bioorganic & medicinal chemistry letters, 10(20), 2255-2257 (2000-10-31)
Structure activity studies on [4-(phenylsulfonyl)phenyl]methylpiperazine led to the discovery of 4-cyclohexyl-alpha-[4-[[4-methoxyphenyl(S)-sufinyl]phenyl]-1-pi perazineacetonitrile, 1, an M2 selective muscarinic antagonist. Affinity at the cloned human M2 receptor was 2.7 nM; the M1/M2 selectivity is 40-fold.
S Yoshihara et al.
Archives of biochemistry and biophysics, 249(1), 8-14 (1986-08-15)
To characterize the properties of diphenyl sulfoxide (DPSO) as a new type of electron acceptor for guinea pig liver aldehyde oxidase (AO), we compared the kinetics of the reductions of DPSO and other classical electron acceptors such as O2 and
Wen-Xian Li et al.
Luminescence : the journal of biological and chemical luminescence, 26(6), 754-761 (2011-05-14)
A novel ternary complex, TbL(5) L'(ClO(4))(3) · 3H(2)O, two binary complexes, TbL(7) (ClO(4))(3) · 3H(2)O and TbL'(3.5) (ClO(4))(3) · 4H(2)O has been synthesized (using diphenyl sulphoxide as the first ligand L, bipyridine as the second ligand L'). Their composition was
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico