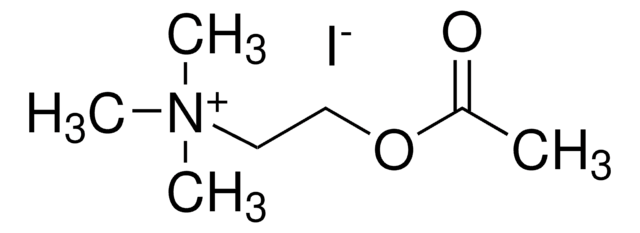
B3253
Butyrylthiocholine iodide
≥98%
Sinónimos:
(2-Mercaptoethyl)trimethylammonium iodide butyrate
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
(CH3)3N(I)CH2CH2SCOCH2CH2CH3
Número de CAS:
Peso molecular:
317.23
Beilstein/REAXYS Number:
3729509
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
≥98%
form
powder
mp
171-174 °C (lit.)
storage temp.
−20°C
SMILES string
[I-].CCCC(=O)SCC[N+](C)(C)C
InChI
1S/C9H20NOS.HI/c1-5-6-9(11)12-8-7-10(2,3)4;/h5-8H2,1-4H3;1H/q+1;/p-1
InChI key
WEQAAFZDJROSBF-UHFFFAOYSA-M
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Butyrylthiocholine iodide is a sulfur-containing analog of butyrylcholine. It is used as a reagent for the determination of butyrylcholinesterase activity.
Application
- Label-Free and Ultrasensitive Detection of Butyrylcholinesterase: A study demonstrated the use of Mn(II)-based electron spin resonance spectroscopy for the ultrasensitive detection of butyrylcholinesterase, using Butyrylthiocholine iodide as a substrate to quantify enzyme activity in the presence of organophosphorus pesticides, crucial for biochemical assay applications (Tang et al., 2022).
- Novel Nanozyme for Biosensing: Research developed a Co, N co-doped porous carbon-based nanozyme, demonstrating its utility as an oxidase mimic for fluorescence and colorimetric biosensing of butyrylcholinesterase, employing Butyrylthiocholine iodide as a key substrate, relevant in enzyme kinetics analysis (Sun et al., 2022).
- Detection System for Anti-Alzheimer′s Drug Screening: A fluorescent platform was constructed using copper nanoclusters and MnO2 nanosheets for the detection of butyrylcholinesterase activity, utilizing Butyrylthiocholine iodide, which may facilitate the screening of anti-Alzheimer′s drugs and probe cholinergic system interactions (Chen et al., 2022).
- Dual-Channel Detection of Butyrylcholinesterase: A study introduced bifunctional metal-organic frameworks with integrated fluorescence and oxidase activities, developed for dual-channel detection of butyrylcholinesterase using Butyrylthiocholine iodide, enhancing methodologies in biochemical assays (Wang et al., 2022).
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Ganiyu Oboh et al.
Neurotoxicology, 77, 12-19 (2019-12-15)
Many plant foods are rich sources of rutin, a flavonoid with many biological activities and health benefits. Exposure to cadmium has been implicated in neurotoxicity and cognitive dysfunction in animal models. However, there is a dearth of information on the
Cristobal Narvaez et al.
Chemosphere, 135, 75-82 (2015-04-29)
Inhibition of blood esterase activities by organophosphate (OP) pesticides has been used as a sensitive biomarker in birds. Furthermore, compared to mammalian vertebrates, less is known about the role of these enzyme activities in the digestive tracts of non-mammalian vertebrates
Gabriela Fernandes et al.
The journal of adhesive dentistry, 22(3), 265-274 (2020-05-22)
To investigate whether dental adhesives modified with polyacrylic acid copper iodide particles could inhibit esterase activity in vitro and the copper release rate from resin matrices, as well as the correlation between the two variables. Different concentrations of copper iodide
You Zhou et al.
Molecules (Basel, Switzerland), 24(23) (2019-11-24)
As there are increased levels and activity of butyrylcholiesterase (BChE) in the late stage of Alzheimer's disease (AD), development of selective BChE inhibitors is of vital importance. In this study, a workflow combining computational technologies and biological assays were implemented
Oya Unsal-Tan et al.
MedChemComm, 10(6), 1018-1026 (2019-07-16)
A novel series of 2-pyrazoline derivatives were designed, synthesized, and evaluated for cholinesterase (ChE) inhibitory, Aβ anti-aggregating and neuroprotective activities. Among these, 3d, 3e, 3g, and 3h were established as the most potent and selective BChE inhibitors (IC50 = 0.5-3.9
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico