930644
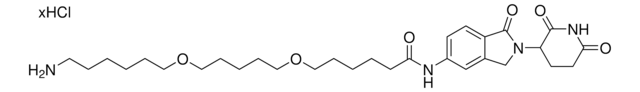
VH 032 amide-PEG2-acid
≥95%
Sinónimos:
(S,R.S)-AHPC-PEG2-acid, (S,R,S)-AHPC-CO-PEG2-C2-acid, 3-[2-(2-{[(2S)-1-[(2S,4R)-4-Hydroxy-2-({[4-(4-methyl-1,3-thiazol-5-yl)phenyl]methyl}carbamoyl)pyrrolidin-1-yl]-3,3-dimethyl-1-oxobutan-2-yl]carbamoyl}ethoxy)ethoxy]propanoic acid, L-Prolinamide, N-[3-[2-(2-carboxyethoxy)ethoxy]-1-oxopropyl]-3-methyl-L-valyl-4-hydroxy-N-[[4-(4-methyl-5-thiazolyl)phenyl]methyl]-, (4R)-
About This Item
Productos recomendados
Application
Technology Spotlight: Degrader Building Blocks for Targeted Protein Degradation
Protein Degrader Building Blocks
Automate your VHL-PEG based PROTACs with Synple Automated Synthesis Platform (SYNPLE-SC002)
Other Notes
Destruction of DNA-Binding Proteins by Programmable Oligonucleotide PROTAC (O′PROTAC): Effective Targeting of LEF1 and ERG
Small-Molecule PROTACS: New Approaches to Protein Degradation
Targeted Protein Degradation: from Chemical Biology to Drug Discovery
Impact of linker length on the activity of PROTACs
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico