930458
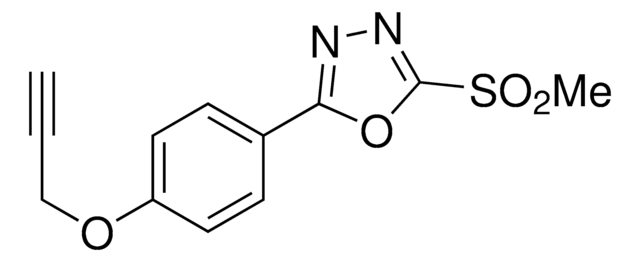
OxMet2-alkyne
Sinónimos:
(3-Phenyl-1,2-oxaziridin-2-yl)(4-(prop-2-yn-1-yloxy)piperidin-1-yl)methanone
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C16H18N2O3
Peso molecular:
286.33
UNSPSC Code:
12352101
NACRES:
NA.22
Productos recomendados
description
Application: Chemoproteomics
Quality Level
form
liquid
storage temp.
−20°C
SMILES string
O=C(N1OC1C2=CC=CC=C2)N(CC3)CCC3OCC#C
Application
OxMet2-alkyne is an oxaziridine probe that can be used to label methionines. A method was developed using cysteine-reactive compounds including this one to allow for unbiased analysis of proteomic data in quantitative applications (Zanon et al. 2021). The method uses light or heavy labelling with the isotopically labelled desthiobiotin azide (isoDTB) tag for mass spectrometry analysis (Zanon et al. 2020). Analysis then uses the isotopic tandem orthogonal proteolysis activity-based protein profiling (isoTOP-ABPP) workflow (Weerapana et al. 2010, Backus et al. 2016)
Other Notes
Profiling the proteome-wide selectivity of diverse electrophiles
A quantitative thiol reactivity profiling platform to analyze redox and electrophile reactive cysteine proteomes
Ethynylation of Cysteine Residues: From Peptides to Proteins in Vitro and in Living Cells
A Chemoproteomic Platform To Assess Bioactivation Potential of Drugs
Inhibition of Zinc-Dependent Histone Deacetylases with a Chemically Triggered Electrophile
Reversibility of Covalent Electrophile-Protein Adducts and Chemical Toxicity
A quantitative thiol reactivity profiling platform to analyze redox and electrophile reactive cysteine proteomes
Ethynylation of Cysteine Residues: From Peptides to Proteins in Vitro and in Living Cells
A Chemoproteomic Platform To Assess Bioactivation Potential of Drugs
Inhibition of Zinc-Dependent Histone Deacetylases with a Chemically Triggered Electrophile
Reversibility of Covalent Electrophile-Protein Adducts and Chemical Toxicity
related product
Referencia del producto
Descripción
Precios
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
Lot/Batch Number
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Profiling the proteome-wide selectivity of diverse electrophiles
Patrick R. A. Zanon, Fengchao Yu, et al
ChemRxiv : the preprint server for chemistry (2021)
De Lin et al.
Chemical research in toxicology, 21(12), 2361-2369 (2009-06-24)
The biotin-tagged electrophiles 1-biotinamido-4-(4'-[maleimidoethylcyclohexane]-carboxamido)butane (BMCC) and N-iodoacetyl-N-biotinylhexylenediamine (IAB) have been used as model electrophile probes in complex proteomes to identify protein targets associated with chemical toxicity. Whereas IAB activates stress signaling and apoptosis in HEK293 cells, BMCC does not. Cysteine
A H El-Khatib et al.
Journal of mass spectrometry : JMS, 52(8), 543-549 (2017-06-04)
1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) derivatives are applied in quantitative proteomics owing to their ability to react with different functional groups, to harbor lanthanoides and hence their compatibility with molecular and elemental mass spectrometry. The new DOTA derivatives, namely Ln-MeCAT-Click and Ln-DOTA-Dimedone
Zarko V Boskovic et al.
ACS chemical biology, 11(7), 1844-1851 (2016-04-12)
Unbiased binding assays involving small-molecule microarrays were used to identify compounds that display unique patterns of selectivity among members of the zinc-dependent histone deacetylase family of enzymes. A novel, hydroxyquinoline-containing compound, BRD4354, was shown to preferentially inhibit activity of HDAC5
Bengt H Gless et al.
The Journal of organic chemistry, 83(17), 10525-10534 (2018-08-07)
The one-pot synthesis and modification of cyclic peptides through a self-cleaving on-resin protocol is described. We apply Dawson's MeDbz linker to achieve direct intramolecular peptide cyclization by thioesterification followed by S → N acyl shift. This native chemical ligation approach
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico