904988
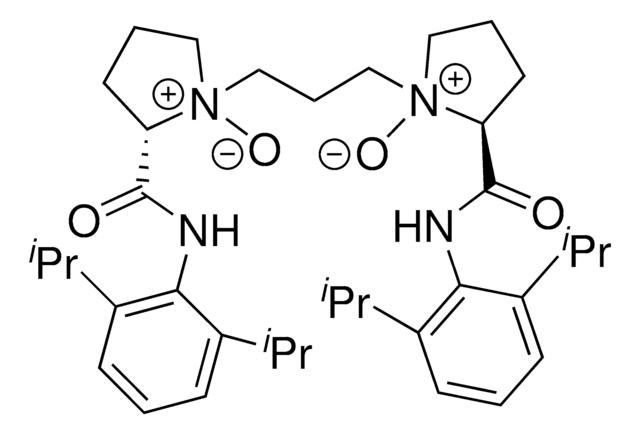
Feng L3-PiPr2
Sinónimos:
(2S,2′S)-1,1′-(propane-1,3-diyl)bis(2-((2,6-diisopropylphenyl)carbamoyl)piperidine-1-oxide)
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C39H60N4O4
Número de CAS:
Peso molecular:
648.92
UNSPSC Code:
12352300
Productos recomendados
form
solid
storage temp.
2-8°C
Application
L3-PiPr2 is a chiral N,N-dioxide ligand developed by the Feng group. In conjunction with a variety of metal salts, this versatile ligand forms and active catalysts complex with application in many different reactions.
Other Notes
An N,N′-Dioxide/In(OTf)3 Catalyst for the Asymmetric Hetero-Diels–Alder Reaction Between Danishefsky′s Dienes and Aldehydes: Application in the Total Synthesis of Triketide
Enantioselective Allylation of Ketones Catalyzed by N,N′-Dioxide and Indium(III) Complex
Asymmetric Dearomatization of Indoles through a Michael/Friedel-Crafts-Type Cascade To Construct Polycyclic Spiroindolines
Enantioselective Allylation of Ketones Catalyzed by N,N′-Dioxide and Indium(III) Complex
Asymmetric Dearomatization of Indoles through a Michael/Friedel-Crafts-Type Cascade To Construct Polycyclic Spiroindolines
Related product
Referencia del producto
Descripción
Precios
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
Lot/Batch Number
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
An N,N'-dioxide/In(OTf)3 catalyst for the asymmetric Hetero-Diels-Alder reaction between Danishefsky's dienes and aldehydes: application in the total synthesis of triketide.
Zhipeng Yu et al.
Angewandte Chemie (International ed. in English), 47(7), 1308-1311 (2008-01-08)
Xin Zhang et al.
The Journal of organic chemistry, 72(14), 5227-5233 (2007-06-15)
Complexes of (S)-pipecolic acid-, L-proline-, and other amino acid-derived N,N'-dioxides coordinated with different metal ions have been investigated in the enantioselective allylation of ketones. A variety of aromatic ketones were found to be suitable substrates in the presence of the
Xiaohu Zhao et al.
Angewandte Chemie (International ed. in English), 54(13), 4032-4035 (2015-02-05)
A highly efficient asymmetric dearomatization of indoles was realized through a cascade reaction between 2-isocyanoethylindole and alkylidene malonates catalyzed by a chiral N,N'-dioxide/Mg(II) catalyst. Fused polycyclic indolines containing three stereocenters were afforded in good yields with excellent diastereo- and enantioselectivities
Hang Zhang et al.
Chemical communications (Cambridge, England), 54(88), 12511-12514 (2018-10-23)
The catalytic asymmetric ene-type reactions of vinylogous hydrazone were accomplished by using chiral N,N'-dioxide-metal salt complexes as catalysts. A wide range of electrophiles, including isatins, α-ketoester, imines, and aldehydes reacted with (E)-2-methyl-N-(piperidin-1-yl)prop-2-en-1-imine efficiently, affording the corresponding homoallylic alcohols and amines
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico



![N-[(1R,2R)-2-(1-Piperidinyl)cyclohexyl]-N′-[4-(trifluoromethyl)phenyl]squaramide 95%](/deepweb/assets/sigmaaldrich/product/structures/238/480/7149c9c0-8769-418a-a96c-77c15dd50cd0/640/7149c9c0-8769-418a-a96c-77c15dd50cd0.png)