560871
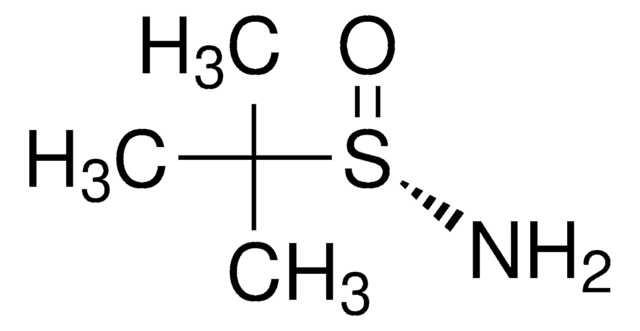
2-Methyl-2-propanesulfinamide
97%, racemic
Sinónimos:
(±)-tert-Butylsulfinamide, 1,1-Dimethylethylsulfinamide, 2-Methyl-2-propanesulfinamide, 2-Methylpropan-2-sulfinamide, tert-Butanesulfinamide, tert-Butylsulfinamide
About This Item
Productos recomendados
Quality Level
assay
97%
form
solid
mp
97-101 °C (lit.)
storage temp.
2-8°C
SMILES string
CC(C)(C)S(N)=O
InChI
1S/C4H11NOS/c1-4(2,3)7(5)6/h5H2,1-3H3
InChI key
CESUXLKAADQNTB-UHFFFAOYSA-N
Application
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Ellman's sulfinamide is available in both enantiomeric and racemic forms for your research. This versatile and useful auxiliary has found extensive use both in academics and industry.
Ellman's sulfinamide is available in both enantiomeric and racemic forms for your research. This versatile and useful auxiliary has found extensive use both in academics and industry.
Ellman's sulfinamide is available in both enantiomeric and racemic forms for your research. This versatile and useful auxiliary has found extensive use both in academics and industry.
Ellman's sulfinamide is available in both enantiomeric and racemic forms for your research. This versatile and useful auxiliary has found extensive use both in academics and industry.
Contenido relacionado
The Ellman group has participated in the development of a variety of C-H functionalization methods. An electron rich phosphine ligand has proven to be very useful for a variety of Rh(I)-catalyzed C-C bond forming reactions applicable to heterocycle synthesis as exemplified in the recent Science paper “Proton Donor Acidity Controls Selectivity in Nonaromatic Nitrogen Heterocycle Synthesis.” Another useful ligand developed for the highly functional group compatible direct arylation of nitrogen heterocycles is described in a 2008 J. Am. Chem. Soc. paper “Rh(I)-Catalyzed Arylation of Heterocycles via C-H Bond Activation: Expanded Scope through Mechanistic Insight.” The Ellman group also developed the chiral amine reagent tert-Butanesulfinamide, which is extensively used in academics and industry for the asymmetric synthesis of amines. A comprehensive survey of tert-Butanesulfinamide methods and applications up through 2009 is provided in the 2010 Chemical Reviews article, “Synthesis and Applications of tert-Butanesulfinamide.”
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico







![(R)-N-[(1R,2R)-2-(3-(3,5-Bis(trifluoromethyl)phenyl)ureido)cyclohexyl]-tert-butyl-sulfinamide 96%](/deepweb/assets/sigmaaldrich/product/structures/389/070/18847164-c6a7-4b4e-abcb-2dbc22493a2d/640/18847164-c6a7-4b4e-abcb-2dbc22493a2d.png)



