539406
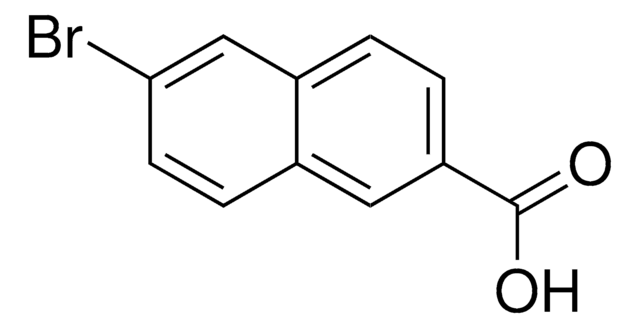
Methyl 6-bromo-2-naphthoate
98%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
BrC10H6CO2CH3
Número de CAS:
Peso molecular:
265.10
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
98%
mp
123-126 °C (lit.)
functional group
bromo
ester
SMILES string
COC(=O)c1ccc2cc(Br)ccc2c1
InChI
1S/C12H9BrO2/c1-15-12(14)10-3-2-9-7-11(13)5-4-8(9)6-10/h2-7H,1H3
InChI key
JEUBRLPXJZOGPX-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Methyl 6-bromo-2-naphthoate undergoes aromatic Finkelstein reaction followed by hydrolysis to afford 6-iodo-2-naphthoic acid.
Application
Methyl 6-bromo-2-naphthoate may be used to synthesize:
- 6-vinyl-2-naphthalencarbaldehyde
- methyl 6-(3-tert-butyl-4-methoxyphenyl)-2-naphthoate
- methyl 6-[3-tert-butyl-4-[(tert-butyldiethylsilyl)oxy]-phenyl]-2-naphthoate
- methyl 6-[3-(1-adamantyl)-4-[(tert-butyldimethylsilyl)-oxy]phenyl]-2-naphthoate
- methyl 6-[3-(1-adamantyl)-4-[[(2,3-dimethyl-1,3-dioxolan-4-yl)methylloxy]phenyl]-2-naphthoate
- 2-bromo-6-(bromomethyl)naphthalene
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Mark W Irvine et al.
Journal of medicinal chemistry, 55(1), 327-341 (2011-11-25)
Competitive N-methyl-d-aspartate receptor (NMDAR) antagonists bind to the GluN2 subunit, of which there are four types (GluN2A-D). We report that some N(1)-substituted derivatives of cis-piperazine-2,3-dicarboxylic acid display improved relative affinity for GluN2C and GluN2D versus GluN2A and GluN2B. These derivatives
Phil M Pithan et al.
Beilstein journal of organic chemistry, 12, 854-862 (2016-06-25)
Cationic biaryl derivatives were synthesized by Suzuki-Miyaura coupling of 3-bromonaphtho[1,2-b]quinolizinium bromide with arylboronic acids. The resulting cationic biaryl derivatives exhibit pronounced fluorosolvatochromic properties. First photophysical studies in different solvents showed that the emission energy of the biaryl derivatives decreases with
Carboxy-1, 4-phenylenevinylene-and carboxy-2, 6-naphthylene-vinylene unsymmetrical substituted zinc phthalocyanines for dye-sensitized solar cells.
Silvestri F, et al.
Journal of Porphyrins and Phthalocyanines, 13(03), 369-375 (2009)
B Charpentier et al.
Journal of medicinal chemistry, 38(26), 4993-5006 (1995-12-22)
The retinoic acid receptors (RARs) transduce retinoid dependant gene regulation, and many biological effects of retinoids are mediated through binding and activation of three closely related receptor subtypes (RAR alpha, RAR beta, and RAR gamma). In order to investigate the
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico



![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)



