480061
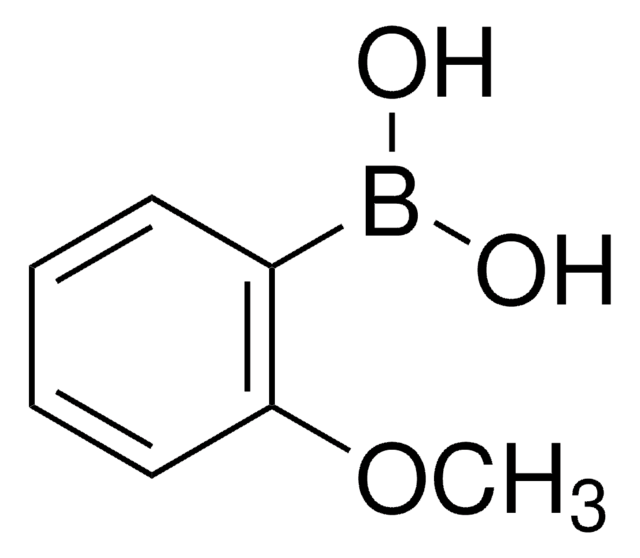
2,6-Dimethylphenylboronic acid
≥95.0%
Sinónimos:
2,6-Dimethylbenzeneboronic acid, 2,6-Xyleneboronic acid, 2,6-Xylylboronic acid
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
(CH3)2C6H3B(OH)2
Número de CAS:
Peso molecular:
149.98
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
≥95.0%
impurities
<10% water
mp
105 °C (dec.) (lit.)
SMILES string
Cc1cccc(C)c1B(O)O
InChI
1S/C8H11BO2/c1-6-4-3-5-7(2)8(6)9(10)11/h3-5,10-11H,1-2H3
InChI key
ZXDTWWZIHJEZOG-UHFFFAOYSA-N
Categorías relacionadas
Application
Reagent used for
Reagent used in Prepration of
- Palladium catalyzed Suzuki-Miyaura coupling reactions
- One-pot ipso-nitration of arylboronic acids including broader substrate scope of heterocycles and functional groups
- Nickel-Catalyzed Cross-Coupling of Chromene Acetals and Boronic Acids
- Visible-light initiated aerobic oxidative hydroxylation catalyzed by Ru-complex
- Rhodium(I)-catalyzed 1,4-addition reactions
- Pd-catalyzed homocouplings
- Expanded scope of Cu assisted Suzuki-Miyaura coupling reactions including aryl chlorides and polyhalo aryl boronates
Reagent used in Prepration of
- Orally bioavialable G Protein-Coupled Receptor 40 agonists for diabetes treatment
- Solid phase synthesis and antitumor structure-activity relationship of Smac triazoloprolines and biarylalanines tetrapeptide libraries
- Protein Kinase inhibitors
Other Notes
contains varying amounts of anhydride
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Sebastian T Le Quement et al.
ACS combinatorial science, 13(6), 667-675 (2011-09-13)
Apoptotic induction mechanisms are of crucial importance for the general homeostasis of multicellular organisms. In cancer the apoptotic pathways are downregulated, which, at least partly, is due to an abundance of inhibitors of apoptosis proteins (IAPs) that block the apoptotic
Santos-Filho, E. F.; et al.
Tetrahedron Letters, 52, 5288-5288 (2011)
Thomas J A Graham et al.
Organic letters, 14(6), 1616-1619 (2012-03-06)
A modular and highly efficient protocol for the synthesis of 2-aryl- and heteroaryl-2H-chromenes is described. Under base-free conditions, readily accessible 2-ethoxy-2H-chromenes undergo C(sp(3))-O activation and C(sp(3))-C bond formation in the presence of an inexpensive nickel catalyst and boronic acids. This
Rhodium(I)-catalyzed 1,4-addition of arylboronic acids to acrylic acid in water: one-step preparation of 3-arylpropionic acids
Vautravers, N. R.; Breit, B.
Synlett, 17, 2517-2520 (2011)
Room-temperature synthesis of tetra-ortho-substituted biaryls by NHC-catalyzed Suzuki-Miyaura couplings.
Linglin Wu et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 17(46), 12886-12890 (2011-10-11)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico






![[Pd(OAc)2]3 reagent grade, 98%](/deepweb/assets/sigmaaldrich/product/structures/508/249/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f/640/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f.png)