126020
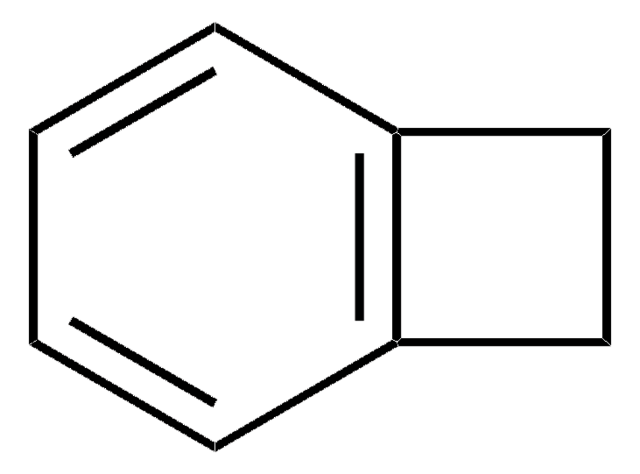
1,4-Dichlorophthalazine
95%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C8H4Cl2N2
Número de CAS:
Peso molecular:
199.04
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
assay
95%
mp
160-162 °C (lit.)
functional group
chloro
storage temp.
−20°C
SMILES string
Clc1nnc(Cl)c2ccccc12
InChI
1S/C8H4Cl2N2/c9-7-5-3-1-2-4-6(5)8(10)12-11-7/h1-4H
InChI key
ODCNAEMHGMYADO-UHFFFAOYSA-N
Categorías relacionadas
Application
1,4-Dichlorophthalazine was used as starting reagent in the synthesis of series of 4-aryl-1-(4-methylpiperazin-1-yl)phthalazines. It was used as coupling reagent in the synthesis of novel soluble polymer-bound ligand. It was often used as building block in medicinal chemistry synthesis.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
Lot/Batch Number
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
A simple and effective soluble polymer-bound ligand for the asymmetric dihydroxylation of olefins: DHQD-PHAL-OPEG-OMe.
Kuang YQ, et al.
Tetrahedron Letters, 42(34), 5925-5927 (2001)
Matthew A J Duncton et al.
Bioorganic & medicinal chemistry letters, 16(6), 1579-1581 (2006-01-03)
A novel class of 1-(isoquinolin-5-yl)-4-arylamino-phthalazines is described as inhibitors of vascular endothelial growth factor receptor II (VEGFR-2). Many compounds display VEGFR-2 inhibitory activity with an IC(50) as low as 0.017 microM in an HTRF enzymatic assay. The compounds also inhibit
K De Wael et al.
Science & justice : journal of the Forensic Science Society, 55(6), 422-430 (2015-12-15)
Reactively-dyed black, navy blue and medium red cotton samples showing metamerism under fluorescent tube illumination were examined. Optical microscopy (bright field, polarization and fluorescence microscopy) was used, followed by microspectrometry in the visible range (MSP Vis), to differentiate the samples
Synthesis of 4-aryl-1-(4-methylpiperazin-1-yl) phthalazines by Suzuki-type cross-coupling reaction.
Guery S, et al.
Synthesis, 2001(05), 699-701 (2001)
William H Bunnelle et al.
Journal of medicinal chemistry, 50(15), 3627-3644 (2007-06-26)
A series of exceptionally potent agonists at neuronal nicotinic acetylcholine receptors (nAChRs) has been investigated. Several N-(3-pyridinyl) derivatives of bridged bicyclic diamines exhibit double-digit-picomolar binding affinities for the alpha 4 beta 2 subtype, placing them with epibatidine among the most
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico