123323
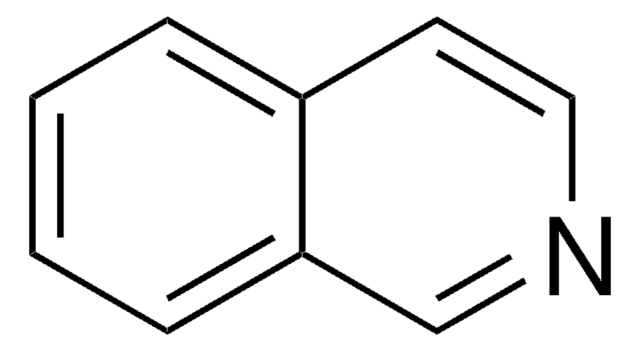
Quinazoline
99%
Sinónimos:
1,3-Benzodiazine, Benzopyrimidine
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C8H6N2
Número de CAS:
Peso molecular:
130.15
Beilstein/REAXYS Number:
109370
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
99%
form
solid
bp
243 °C (lit.)
mp
46-48 °C (lit.)
solubility
H2O: freely soluble
organic solvents: soluble
SMILES string
c1ccc2ncncc2c1
InChI
1S/C8H6N2/c1-2-4-8-7(3-1)5-9-6-10-8/h1-6H
InChI key
JWVCLYRUEFBMGU-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Quinazolines has applications in medicinal chemistry due to their antibacterial, antifungal, anticonvulsant, anti-inflammatory and antitumor activities. It is the basic structural unit of pharmaceuticals and plays an important role in modern synthesis of antitumor drugs.
Application
Quinazoline was used to study the electrochemical behaviour of quinazoline using modern polarographic and voltammetric methods.
Biochem/physiol Actions
Genotoxicity of quinazoline was established by bacterial SOS Chromotest (Escherichia Coli).
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
222.8 °F - closed cup
flash_point_c
106 °C - closed cup
ppe
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Kunal Nepali et al.
European journal of medicinal chemistry, 196, 112291-112291 (2020-04-24)
This study reports the design, synthesis and evaluation of a series of histone deacetylase (HDAC) inhibitors containing purine/purine isoster as a capping group and an N-(2-aminophenyl)-benzamide unit. In vitro cytotoxicity studies reveal that benzamide 14 suppressed the growth of triple-negative breast
Bruna Possato et al.
Dalton transactions (Cambridge, England : 2003), 46(24), 7926-7938 (2017-06-13)
We report on the investigation of a new series of symmetric trinuclear ruthenium complexes combined with azanaphthalene ligands: [Ru
Reddy Amala et al.
BioImpacts : BI, 11(1), 15-22 (2021-01-21)
Introduction: Inflammation is the primary response caused due to harmful stimuli which are followed by the increased draining of plasma and immune cells from the body into the site of the injured tissue. A signaling cascade of growth factors and
Polarographic and voltammetric determination of quinazoline-the structural unit of anticancer drugs.
Hladikova J, et al.
Sensing in Electroanalysis, 3, 165-175 (2008)
Elham Bagheri et al.
Current pharmaceutical design, 24(13), 1395-1404 (2018-02-01)
Quinazoline is an aromatic bicyclic compound exhibiting several pharmaceutical and biological activities. This study was conducted to investigate the potential wound healing properties of Synthetic Quinazoline Compound (SQC) on experimental rats. The toxicity of SQC was determined by MTT cell
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico