21590
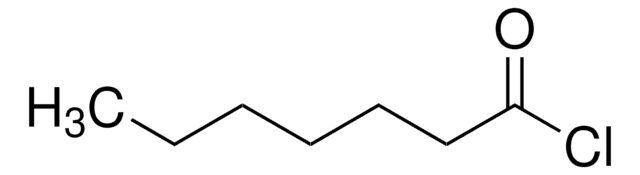
Hexanoyl chloride
purum, ≥98.0% (GC)
Synonym(s):
Caproyl chloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3(CH2)4COCl
CAS Number:
Molecular Weight:
134.60
Beilstein:
506332
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
purum
Quality Level
Assay
≥98.0% (GC)
form
liquid
refractive index
n20/D 1.426 (lit.)
n20/D 1.426
bp
150-153 °C (lit.)
solubility
chloroform: soluble(lit.)
diethyl ether: soluble(lit.)
density
0.963 g/mL at 25 °C (lit.)
functional group
acyl chloride
SMILES string
CCCCCC(Cl)=O
InChI
1S/C6H11ClO/c1-2-3-4-5-6(7)8/h2-5H2,1H3
InChI key
YWGHUJQYGPDNKT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Hexanoyl chloride has been used in the synthesis of:
- (±)-7-butyl-6,8-dihydroxy-3-pentyl-3,4-dihydroisochromen-1-one
- 14-methyl-1-octadecene, the sex pheromone of the peach leafminer moth
- hexanoyl-coated nanofibers dispersible in several organic solvents
- natural isocarbostyril ruprechstyril (3-n-pentyl-6-methoxy-8-hydroxy-1(2H)-isoquinolinone), isolated from Ruprechtia tangarana
- 5-chloro-8-hydroxy-6-methoxy-3-pentylisocoumarin, the 5-chloro analog of naturally occurring 7-chloro-8-hydroxy-6-methoxy-3-pentylisocoumarin, isolated from Tessmannia densiflora
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Flam. Liq. 3 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
122.0 °F - closed cup
Flash Point(C)
50 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Sanna Virtanen et al.
Carbohydrate polymers, 177, 105-115 (2017-10-01)
Using softwood pulp as the starting material, the synthesis of regioselectively substituted mixed cellulose esters with varying degree of substitution and ratio of short/long chains was successfully completed. The structures of the cellulose esters were characterised. The impact of the
Aamer Saeed
Journal of Asian natural products research, 13(6), 505-511 (2011-05-31)
The synthesis of title isocoumarin, the 5-chloro analog of naturally occurring 7-chloro-8-hydroxy-6-methoxy-3-pentylisocoumarin, isolated from Tessmannia densiflora is described. Chlorination of ethyl 2-(2-ethoxy-2-oxoethyl)-4,6-dimethoxybenzoate (2) afforded 3-chloro ester (3) followed by hydrolysis to furnish the 2-(carboxymethyl)-3-chloro-4,6-dimethoxybenzoic acid (4) that was converted to
Tao Zhang et al.
Molecules (Basel, Switzerland), 18(5), 5201-5208 (2013-05-09)
An asymmetric synthesis of 14-methyl-1-octadecene, the sex pheromone of the peach leafminer moth has been achieved. The target molecule was synthesized in six linear steps and in 30.3% overall yield from commercially available hexanoyl chloride, (S)-4-benzyloxazolidin-2-one and 1,9-nonanediol. The hexanoyl
Aamer Saeed
Natural product research, 27(13), 1153-1158 (2012-08-14)
A short total synthesis of natural isocarbostyril ruprechstyril (3-n-pentyl-6-methoxy-8-hydroxy-1(2H)-isoquinolinone) isolated from Ruprechtia tangarana is reported. 6,8-Dimethoxy-3-pentylisocoumarin obtained by condensation of 3,5-dimethoxyhomophthalic anhydride with hexanoyl chloride was smoothly converted to O-methylruprechstyril by refluxing with methanamide. Regioselective demethylation of the latter using
Aamer Saeed et al.
Journal of Asian natural products research, 15(10), 1112-1122 (2013-07-23)
A new total synthesis of ( ± )-7-butyl-6,8-dihydroxy-3-pentyl-3,4-dihydroisochromen-1-one, isolated as R-enantiomer from Geotrichum sp., has been described. Reaction of 4-butyl-3,5-dimethoxyhomophthalic anhydride with hexanoyl chloride in the presence of 1,1,3,3-tetramethyl guanidine and triethyl amine afforded 7-butyl-6,8-dimethoxy-3-pentylisochromen-1-one, which was converted into corresponding 3,4-dihydroisochromen-1-one by
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service