D7600
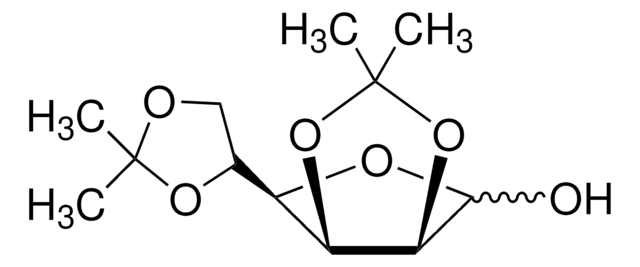
1,2:5,6-Di-O-isopropylidene-α-D-glucofuranose
98%
Synonym(s):
1,2,5,6-diisopropylidene-D-glucose, D-Glucose diacetonide, Diacetone-D-glucose
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C12H20O6
CAS Number:
Molecular Weight:
260.28
Beilstein:
84386
EC Number:
MDL number:
UNSPSC Code:
12352201
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
powder
optical activity
[α]20/D −18°, c = 1% in H2O
mp
109-113 °C (lit.)
SMILES string
[H][C@@]1(O[C@H]2O[C@@H](O[C@@H]2[C@H]1O)C(Cl)(Cl)Cl)[C@H]3COC(C)(C)O3
InChI
1S/C12H20O6/c1-11(2)14-5-6(16-11)8-7(13)9-10(15-8)18-12(3,4)17-9/h6-10,13H,5H2,1-4H3/t6-,7+,8-,9-,10-/m1/s1
InChI key
KEJGAYKWRDILTF-JDDHQFAOSA-N
Application
1,2:5,6-Di-O-isopropylidene-α-D-glucofuranose can be used as a starting material to prepare:
- Biologically active L-acovenose, 6-deoxy-L-idose and, carbanucleoside enantiomers.
- Vinyl ether-based chiral carbohydrate synthon by reacting with acetylene using superbase catalytic systems.
- Fluoro-thiofuranosyl nucleosides of biological importance.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Carbanucleosides: synthesis of both enantiomers of 2-(6-chloro-purin-9-yl)-3, 5-bishydroxymethyl cyclopentanol from d-glucose
Roy BG, et al.
Tetrahedron Letters, 47(50), 8821-8825 (2006)
Direct vinylation of glucose derivatives with acetylene
Trofimov BA, et al.
Tetrahedron, 63(47), 11661-11665 (2007)
An efficient synthesis of 3-fluoro-5-thio-xylofuranosyl nucleosides of thymine, uracil, and 5-fluorouracil as potential antitumor or/and antiviral agents
Tsoukala E, et al.
Bioorganic & Medicinal Chemistry, 15(9), 3241-3247 (2007)
T Z Csáky
Journal of medicine, 16(5-6), 575-586 (1985-01-01)
Based on a previous observation, it was postulated that dimethylsulfoxide (DMSO) acts as a carrier-model in the intestinal absorption of glucose and galactose (Csáky and Ho, 1966). It was further hypothetized that DMSO forms a loosely-bonded hydrophobic complex with the
E White et al.
Biomedical mass spectrometry, 9(9), 395-405 (1982-09-01)
Two isotope dilution mass spectrometric methods have been developed for the determination of D-glucose in human serum. Each uses a uniformly labeled (13C)glucose as the internal standard. The first method involves conversion of glucose into 1,2:5,6-di-O-isopropylidene-alpha-D-glucofuranose and an extensive clean-up
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![(3AR,5S,6S,6AR)-5-((R)-2,2-DIMETHYL-1,3-DIOXOLAN-4-YL)-2,2-DIMETHYLTETRAHYDROFURO[3,2-D][1,3]DIOXOL-6-OL AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/241/825/1c695a85-5c36-42d3-806a-30876a4dabac/640/1c695a85-5c36-42d3-806a-30876a4dabac.png)