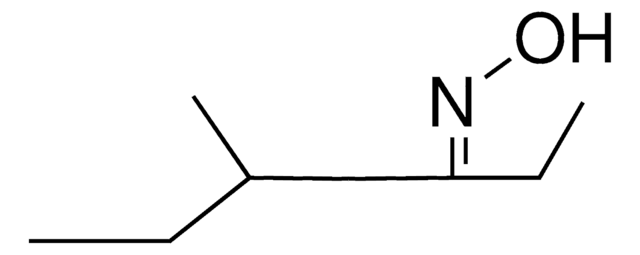
D7151
1,7-Dioxaspiro[5.5]undecane
≥97%
Synonym(s):
Olive-fly ketal
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H16O2
CAS Number:
Molecular Weight:
156.22
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥97%
form
liquid
refractive index
n20/D 1.464 (lit.)
bp
193 °C/750 mmHg (lit.)
density
1.02 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
C1CCC2(CCCCO2)OC1
InChI
1S/C9H16O2/c1-3-7-10-9(5-1)6-2-4-8-11-9/h1-8H2
InChI key
GBBVHDGKDQAEOT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Biochem/physiol Actions
Pheromone for Dacus oleae
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
147.2 °F - closed cup
Flash Point(C)
64 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Mary T Fletcher et al.
Chemical communications (Cambridge, England), (12)(12), 1302-1303 (2002-07-12)
The demonstration that both oxygen atoms of 1,7-dioxaspiro[5.5]undecane (1), the sex-pheromone of the female olive fly, originate from dioxygen, strongly implicates monooxygenase mediated processes in assembly of (1), and reveals unexpected complexity in the formation of its nine-carbon precursor.
Anat Levi-Zada et al.
Journal of chemical ecology, 38(8), 1036-1041 (2012-09-04)
The olive fruit fly, Bactrocera oleae (Diptera: Tephritidae), uses 1,7-dioxaspiro[5.5]undecane ("olean"), produced primarily by females, as a sex pheromone. We used sequential solid phase microextraction-gas chromatography mass spectrometry (SPME-GCMS) analysis to show that female olive flies release about 1000 ng
Brett D Schwartz et al.
Organic letters, 7(6), 1173-1176 (2005-03-12)
[reaction: see text] A biosynthetic scheme rationalizing the formation of (+/-)-1,7-dioxaspiro[5.5]undecane (5) in the fruit fly species Bactrocera cacuminata and Bactrocera oleae (olive fruit fly) is presented. Incorporation studies with deuterium-labeled keto aldehyde (10), 1,5-nonanediol (11), and 1,5,9-nonanetriol (12), and
N Rysanek et al.
Acta crystallographica. Section C, Crystal structure communications, 52 ( Pt 11), 2932-2936 (1996-11-15)
The complex beta-cyclodextrin-1,7-dioxaspiro[5,5]undecane nonahydrate, C42H70O35.C9H16O2.9H2O, belongs to the class of beta-cyclodextrin dimeric-type complexes. The racemic guest molecule is present in a disordered position. Both enantiomers are located in two different regions inside the channel formed by the host dimers.
S Makedonopoulou et al.
Acta crystallographica. Section B, Structural science, 57(Pt 3), 399-409 (2001-05-25)
The enantiomers of racemic olive fly sex pheromone 1,7-dioxaspiro[5.5]undecane (1) have been isolated by crystallization with enantiospecific cyclodextrin hosts: (S)-(1) crystallizes with heptakis(2,3,6-tri-O-methyl)-beta-cyclodextrin (TMbetaCD) and (R)-(1) with hexakis(2,3,6-tri-O-methyl)-alpha-cyclodextrin (TMalphaCD). The crystal structure of TMbetaCD/(S)-(1) from synchrotron radiation data at 100
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![3-Oxabicyclo[3.1.0]hexane-2,4-dione 98%](/deepweb/assets/sigmaaldrich/product/structures/775/685/428f7212-fd69-42ea-bf55-57f32b86de70/640/428f7212-fd69-42ea-bf55-57f32b86de70.png)