59539
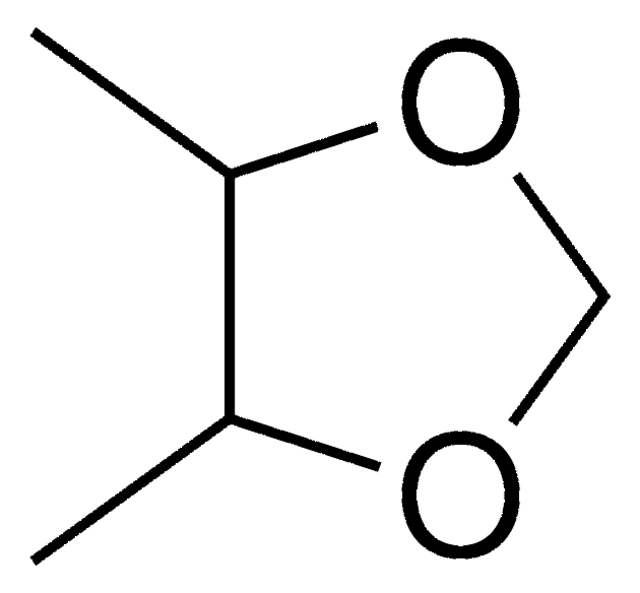
(4S,5S)-2,2-Dimethyl-1,3-dioxolane-4,5-dimethanol
≥97.0% (sum of enantiomers, GC)
Synonym(s):
(+)-2,3-O-Isopropylidene-L-threitol, (4S,5S)-4,5-Bis(hydroxymethyl)-2,2-dimethyl-1,3-dioxolane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C7H14O4
CAS Number:
Molecular Weight:
162.18
Beilstein:
1280797
EC Number:
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥97.0% (sum of enantiomers, GC)
form
solid
optical activity
[α]20/D +3.0±0.5°, c = 5% in ethanol
bp
92-94 °C/0.1 mmHg (lit.)
mp
46-50 °C
functional group
ether
hydroxyl
ketal
SMILES string
CC1(C)O[C@@H](CO)[C@H](CO)O1
InChI
1S/C7H14O4/c1-7(2)10-5(3-8)6(4-9)11-7/h5-6,8-9H,3-4H2,1-2H3/t5-,6-/m0/s1
InChI key
INVRLGIKFANLFP-WDSKDSINSA-N
Application
(4S,5S)-2,2-Dimethyl-1,3-dioxolane-4,5-dimethanol can be used:
- As a key intermediate for the preparation of (6Z)-cis-9S,10R-epoxy-nonadecene, a female sex pheromone of elm spanworm.
- To prepare a chiral diphosphite ligand by reacting with phosphorochloridite using excess of NEt3.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis of chiral alkenyl epoxides: the sex pheromone of the elm spanworm Ennomus subsignaria (Hubner)(Lepidoptera: Geometridae)
MaGee DI, et al.
Tetrahedron, 67(29), 5329-5338 (2011)
Modular chiral diphosphite derived from l-tartaric acid. Applications in metal-catalyzed asymmetric reactions
Rosas-Hern'andez A, et al.
J. Mol. Catal. A: Chem., 328(1-2), 68-75 (2010)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service