47552
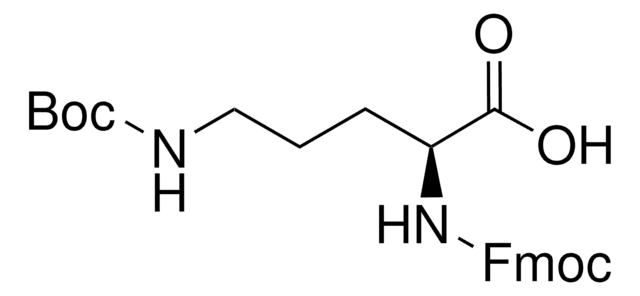
Fmoc-Dap-OH
≥97.0% (HPLC)
Synonym(s):
Nα-Fmoc-L-2,3-diaminopropionic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C18H18N2O4
CAS Number:
Molecular Weight:
326.35
Beilstein:
7658592
MDL number:
UNSPSC Code:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.26
Recommended Products
Quality Level
Assay
≥97.0% (HPLC)
form
solid
reaction suitability
reaction type: Fmoc solid-phase peptide synthesis
application(s)
peptide synthesis
functional group
Fmoc
storage temp.
2-8°C
SMILES string
NC[C@H](NC(=O)OCC1c2ccccc2-c3ccccc13)C(O)=O
InChI
1S/C18H18N2O4/c19-9-16(17(21)22)20-18(23)24-10-15-13-7-3-1-5-11(13)12-6-2-4-8-14(12)15/h1-8,15-16H,9-10,19H2,(H,20,23)(H,21,22)/t16-/m0/s1
InChI key
HDSLKWZYHRLRRL-INIZCTEOSA-N
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Ahmet Kertmen et al.
Langmuir : the ACS journal of surfaces and colloids, 35(15), 5281-5293 (2019-03-27)
Numerous glutamine analogues have been reported as irreversible inhibitors of the glucosamine-6-phosphate (GlcN-6-P) synthase in pathogenic Candida albicans in the last 3.5 decades. Among the reported inhibitors, the most effective N3-(4-methoxyfumaroyl)-l-2,3-diaminopropanoic acid (FMDP) has been extensively studied in order to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service