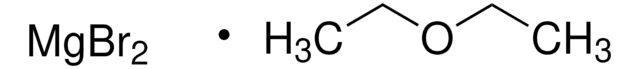360074
Magnesium bromide
98%
Synonym(s):
Ddibromomagnesium, Magnesium bromide salt, Magnesium dibromide
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
MgBr2
CAS Number:
Molecular Weight:
184.11
EC Number:
MDL number:
UNSPSC Code:
12352302
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
powder
mp
711 °C (lit.)
density
3.72 g/mL at 25 °C (lit.)
SMILES string
Br[Mg]Br
InChI
1S/2BrH.Mg/h2*1H;/q;;+2/p-2
InChI key
OTCKOJUMXQWKQG-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
Application
Magnesium bromide (MgBr2) can be used as a catalyst in:
- Acetylation and benzoylation of alcohols (primary and secondary) with acid anhydrides.
- In the synthesis of dihydropyrimidinones , trifluoromethylnaphthalenes , substituted 1,2,3,4 tetrahydropyrimidin-2-ones .
- In the preparation of Aldol derivatives by hydrogenation of Baylis-Hillman olefins.
- Deprotection of SEM (2-(Trimethylsilyl)ethoxymethyl) ether group.
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Magnesium bromide mediated highly diastereoselective heterogeneous hydrogenation of olefins
Bouzide, Abderrahim
Organic Letters, 4(8), 1347-1350 (2002)
Synthesis of trifluoromethylnaphthalenes
Mellor JM, et al.
Tetrahedron, 56(51), 10067-10074 (2000)
Magnesium bromide catalysed acylation of alcohols
Pansare SV, et al.
Synthetic Communications, 30(14), 2587-2592 (2000)
A facile and efficient one-pot synthesis of dihydropyrimidinones catalyzed by magnesium bromide under solvent-free conditions
Salehi H and Guo Q-X
Synthetic Communications, 34(1), 171-179 (2004)
Novel deprotection of SEM ethers: a very mild and selective method using magnesium bromide
Vakalopoulos A and Hoffmann HMR
Organic Letters, 2(10), 1447-1450 (2000)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service