294535
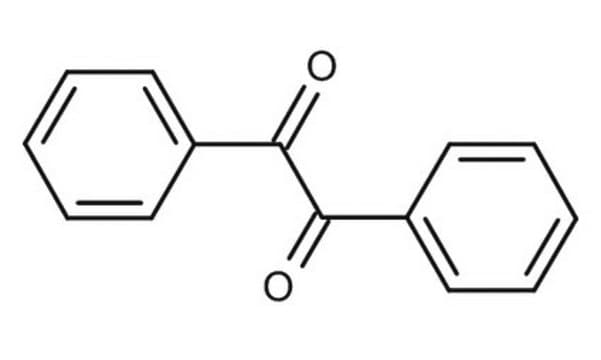
meso-Hydrobenzoin
99%
Synonym(s):
meso-1,2-Diphenyl-1,2-ethanediol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5CH(OH)CH(OH)C6H5
CAS Number:
Molecular Weight:
214.26
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
solid
mp
137-139 °C (lit.)
solubility
ethanol: soluble 25 mg/mL, clear, colorless
functional group
hydroxyl
phenyl
SMILES string
O[C@H]([C@H](O)c1ccccc1)c2ccccc2
InChI
1S/C14H14O2/c15-13(11-7-3-1-4-8-11)14(16)12-9-5-2-6-10-12/h1-10,13-16H/t13-,14+
InChI key
IHPDTPWNFBQHEB-OKILXGFUSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Desymmetrization of meso-hydrobenzoin using chiral phosphine catalyst has been reported. Conversion of meso-hydrobenzoin to trans-stillbene oxide by treatment with an aryl sulfonyl chloride and aqueous sodium hydroxide has been reported.
Application
meso-Hydrobenzoin has been used in the preparation of trans-methyl meso-hydrobenzoin phosphite.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Conversion of meso-Hydrobenzoin with Arylsulfonyl Chlorides and Base to trans-Stilbene Oxide and to 1, 1-Diphenyl-2-(p-toluenesulfonyloxy) ethylene1.
Curtin DY and Hendrickson YG.
The Journal of Organic Chemistry, 21(11), 1260-1263 (1956)
E Vedejs et al.
The Journal of organic chemistry, 69(4), 1389-1392 (2004-02-14)
The desymmetrization of meso-hydrobenzoin is described using chiral phosphine catalysts 8b-d and 9-11. The best enantioselectivity at room temperature was obtained with the newly synthesized phospholane 8c and benzoic anhydride, but the reaction is very slow. Much faster reactions, but
Preparation and crystal structures of trans-methyl meso-hydrobenzoin phosphite and phosphate.
Newton MG and Campbell BS.
Journal of the American Chemical Society, 96(25), 7790-7797 (1974)
Chromatograms
application for HPLCapplication for HPLCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service