All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H8OS
CAS Number:
Molecular Weight:
116.18
Beilstein:
106464
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
crystals
mp
60-64 °C (lit.)
functional group
ketone
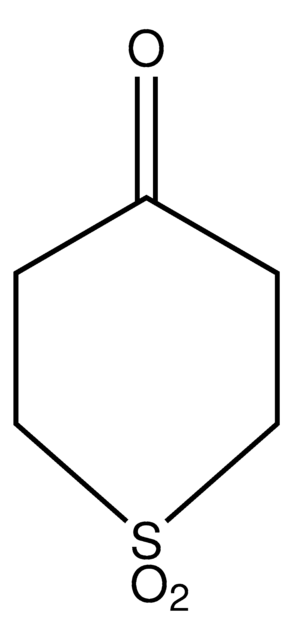
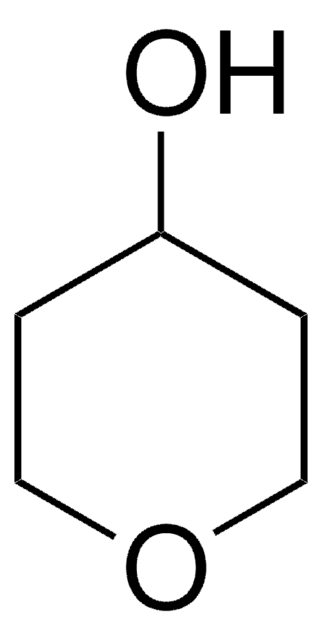
SMILES string
O=C1CCSCC1
InChI
1S/C5H8OS/c6-5-1-3-7-4-2-5/h1-4H2
InChI key
OVRJVKCZJCNSOW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
The diastereoselectivity of the aldol reaction of tetrahydro-4H-thiopyran-4-one has been studied.
Application
Tetrahydro-4H-thiopyran-4-one was used in the preparation of meso 1,9-diketones.
The product has been utilized in various condensation reactions for the preparation of dipeptides, spiroimidazolones, and tetrahydrocarbazoles and α-hydroxy esters.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synlett, 1605-1605 (2007)
Journal of Heterocyclic Chemistry, 30, 81-81 (1993)
Journal of Heterocyclic Chemistry, 31, 397-397 (1994)
Dale E Ward et al.
Organic letters, 8(12), 2631-2634 (2006-06-02)
Meso 1,9-diketones (six to seven stereocenters) are readily obtained by stepwise or simultaneous two-directional aldol reactions of tetrahydro-4H-thiopyran-4-one with a thiopyran-derived aldehyde or dialdehyde. Enantioselective enolizations of these diketones with the lithium amide from (R,R)-bis(1-phenylethyl)amine occur with simultaneous kinetic resolution
Dale E Ward et al.
The Journal of organic chemistry, 67(5), 1618-1629 (2002-03-02)
The diastereoselectivity of the aldol reaction of tetrahydro-4H-thiopyran-4-one (3) with 1,4-dioxa-8-thiaspiro[4.5]decane-6-carboxaldehyde (9a) under a variety of conditions is examined. Under optimized conditions, three of the four possible diastereomers from this aldol reaction can be obtained selectively (3-16:1). Reactions of 9a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service