116122
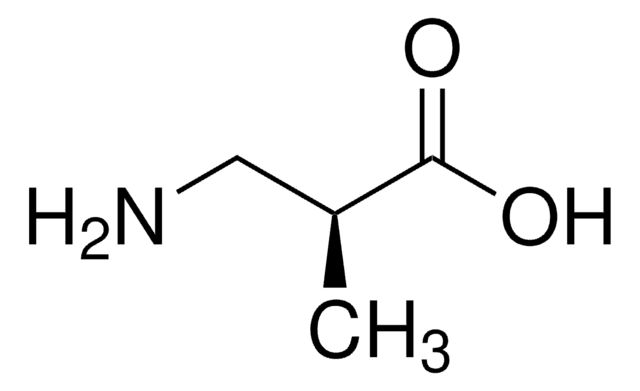
D-2-Aminobutyric acid
98%
Synonym(s):
(R)-(−)-2-Aminobutyric acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
C2H5CH(NH2)CO2H
CAS Number:
Molecular Weight:
103.12
Beilstein:
1720934
EC Number:
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
optical activity
[α]20/D −21 to −19°, c = 4 in H2O
optical purity
ee: 98% (GLC)
reaction suitability
reaction type: solution phase peptide synthesis
mp
>300 °C (lit.)
application(s)
peptide synthesis
SMILES string
CC[C@@H](N)C(O)=O
InChI
1S/C4H9NO2/c1-2-3(5)4(6)7/h3H,2,5H2,1H3,(H,6,7)/t3-/m1/s1
InChI key
QWCKQJZIFLGMSD-GSVOUGTGSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Chen Qi et al.
Polymers, 12(9) (2020-09-13)
Eight kinds of chiral diacid monomers were prepared with amino acids with different side groups or configurations. Polyester-imides (PEIs) were synthesized from these diacid monomers and diphenol monomers through polycondensation reaction, and the performances and properties were compared with the
Quantification of aminobutyric acids and their clinical applications as biomarkers for osteoporosis.
Zhiying Wang et al.
Communications biology, 3(1), 39-39 (2020-01-24)
Osteoporosis is a highly prevalent chronic aging-related disease that frequently is only detected after fracture. We hypothesized that aminobutyric acids could serve as biomarkers for osteoporosis. We developed a quick, accurate, and sensitive screening method for aminobutyric acid isomers and
Site-selective traceless Staudinger ligation for glycoprotein synthesis reveals scope and limitations.
Gonçalo J L Bernardes et al.
Chembiochem : a European journal of chemical biology, 12(9), 1383-1386 (2011-05-21)
Tatsuo Yajima et al.
Bioscience, biotechnology, and biochemistry, 71(5), 1338-1341 (2007-05-09)
An attempt was made to use a simple procedure to obtain (R)- and (S)-2-aminobutanoic acids [(R)- and (S)-1] which are non-proteinogenic alpha-amino acids and are useful as chiral reagents in asymmetric syntheses. Compound (RS)-1 p-toluenesulfonate [(RS)-2], which is known to
Jong Jin Lee et al.
Nuclear medicine and biology, 39(3), 325-333 (2011-12-06)
We evaluated new (111)In-labeled amino acid derivatives, in which the amino acids are conjugated with1,4,7,10-tetra-azacyclododecane-1,4,7,10-tetraacetic acid (DOTA), 1,4,7,10-tetraazacyclododecane-1,7-diacetic acid (DO2A) or 1,4,7,10-tetraazacyclododecane-1,4,7-triacetic acid (DO3A). DOTA-aminoalanine (DOTA-A), DOTA-aminohomoalanine (DOTA-H), DOTA-lysine (DOTA-L), DO2A-alanine (DO2A-A), DO3A-alanine (DO3A-A) and DO3A-homoalanine (DO3A-H) were labeled with
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service