88580
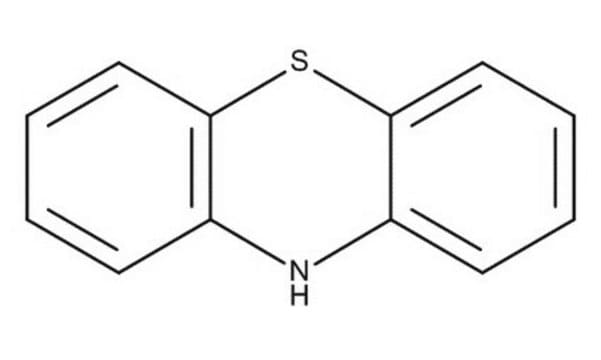
Phenothiazine
purum, ≥98.0% (GC)
Synonym(s):
PTZ, 10H-Phenothiazine
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Empirical Formula (Hill Notation):
C12H9NS
CAS Number:
Molecular Weight:
199.27
Beilstein:
143237
EC Number:
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
grade
purum
Quality Level
Assay
≥98.0% (GC)
form
pellets
bp
371 °C (lit.)
mp
182-187 °C (lit.)
183-187 °C
SMILES string
N1c2ccccc2Sc3ccccc13
InChI
1S/C12H9NS/c1-3-7-11-9(5-1)13-10-6-2-4-8-12(10)14-11/h1-8,13H
InChI key
WJFKNYWRSNBZNX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
The structure of phenothiazine is rigid, being tricyclic. It is known to alter dopamine (3,4-dihydroxyphenethylamine). Its use as an electron donor is based on its unique hole transporting ability, electron releasing nitrogen and sulfur heteroatoms and its non-planar structure leading to lower molecular aggregation.
Application
Phenothiazine finds uses in metal free organic dye sensitizers, dyes and antioxidants.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Skin Sens. 1 - STOT RE 2 Oral
Target Organs
Blood
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Acridine and phenothiazine derivatives as pharmacotherapeutics for prion disease
Carsten K, et al
Proceedings of the National Academy of Sciences of the USA, 98(17), 9834-9841 (2001)
Photodegradation of trimeprazine triggered by self-photogenerated singlet molecular oxygen
Waseem A, et al
Journal of Saudi Chemical Society (2012)
Asif M, et al
Arabian Journal of Chemistry null
A S Horn et al.
Proceedings of the National Academy of Sciences of the United States of America, 68(10), 2325-2328 (1971-10-01)
Phenothiazines and butyrophenones are known to alter dopamine (3,4-dihydroxyphenethylamine) metabolism in the brain in a fashion suggesting that they may block dopamine receptors. We observed, using Dreiding molecular models, that dopamine in its solid-state conformation is superimposable upon a portion
Dose dependences of lipid microviscosity of biological membranes induced by synthetic antioxidant potassium phenosan salt.
N P Palmina et al.
Doklady. Biochemistry and biophysics, 443, 100-104 (2012-05-09)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service












![5H-Dibenz[b,f]azepine 97%](/deepweb/assets/sigmaaldrich/product/structures/396/216/18f00414-a76e-46d7-90cf-820ad902e559/640/18f00414-a76e-46d7-90cf-820ad902e559.png)