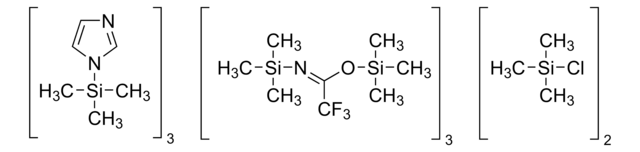
15209
N,O-Bis(trimethylsilyl)trifluoroacetamide with trimethylchlorosilane
for GC derivatization, LiChropur™, contains 10% TMCS, 98% (excluding TMCS)
Synonym(s):
BSTFA + TMCS
About This Item
Recommended Products
grade
for GC derivatization
Quality Level
Assay
≥97.5% (excluding TMCS, GC)
98% (excluding TMCS)
form
liquid
quality
LiChropur™
reaction suitability
reagent type: derivatization reagent
reaction type: Silylations
technique(s)
gas chromatography (GC): suitable
impurities
9.0-11.0% TMCS
refractive index
n20/D 1.384 (lit.)
bp
45-50 °C/14 mmHg (lit.)
density
0.97 g/mL (lit.)
SMILES string
C[Si](C)(C)O\C(=N\[Si](C)(C)C)C(F)(F)F
InChI
1S/C8H18F3NOSi2/c1-14(2,3)12-7(8(9,10)11)13-15(4,5)6/h1-6H3/b12-7+
InChI key
XCOBLONWWXQEBS-KPKJPENVSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Determination of Δ(9)-tetrahydrocannabinol, 11-nor-carboxy-Δ(9)-tetrahydrocannabinol and cannabidiol in human plasma and urine after a commercial cannabidiol oil product intake.: This study uses N,O-Bis(trimethylsilyl)trifluoroacetamide with trimethylchlorosilane for derivatization to enhance the sensitivity and accuracy of cannabinoid detection in biological samples. This method is vital for forensic toxicology and clinical research, providing reliable data for cannabinoid analysis (Papoutsis et al., 2024).
- The impact of lipid degradation on fingerprint quality on fired firearm cartridges.: This research employs N,O-Bis(trimethylsilyl)trifluoroacetamide with trimethylchlorosilane to study lipid degradation and its effect on fingerprint quality on firearm cartridges. The findings are crucial for forensic science, particularly in improving methods for preserving and analyzing fingerprint evidence (Goulart et al., 2023).
- Contaminants of emerging concern: Silylation procedures, evaluation of the stability of silyl derivatives and associated measurement uncertainty.: This paper evaluates the use of N,O-Bis(trimethylsilyl)trifluoroacetamide with trimethylchlorosilane in silylation procedures for environmental analysis. The study assesses the stability and measurement uncertainty of silyl derivatives, which is crucial for accurate monitoring of environmental contaminants (Ljoncheva et al., 2023).
- Screening of Organic Acidurias by Gas Chromatography-Mass Spectrometry (GC-MS).: This research utilizes N,O-Bis(trimethylsilyl)trifluoroacetamide with trimethylchlorosilane in the derivatization process for screening organic acidurias. This method is significant for clinical diagnostics, providing a reliable approach for detecting metabolic disorders (Scott et al., 2022).
- A sensitive, robust method for determining natural and synthetic hormones in surface and wastewaters by continuous solid-phase extraction-gas chromatography-mass spectrometry.: The study presents a method using N,O-Bis(trimethylsilyl)trifluoroacetamide with trimethylchlorosilane for the detection of hormones in environmental samples. This robust method is essential for monitoring water quality and detecting endocrine-disrupting compounds (Chafi and Ballesteros, 2022).
Other Notes
Legal Information
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1A
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
15.8 °F - closed cup
Flash Point(C)
-9 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Results of a study involving the ability few Fluka silylating reagents to form GC-MS-compatible trimethylsilylmethyl derivatives of NSAIDs
Results of a study involving the ability few Fluka silylating reagents to form GC-MS-compatible trimethylsilylmethyl derivatives of NSAIDs
Results of a study involving the ability few Fluka silylating reagents to form GC-MS-compatible trimethylsilylmethyl derivatives of NSAIDs
Results of a study involving the ability few Fluka silylating reagents to form GC-MS-compatible trimethylsilylmethyl derivatives of NSAIDs
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service