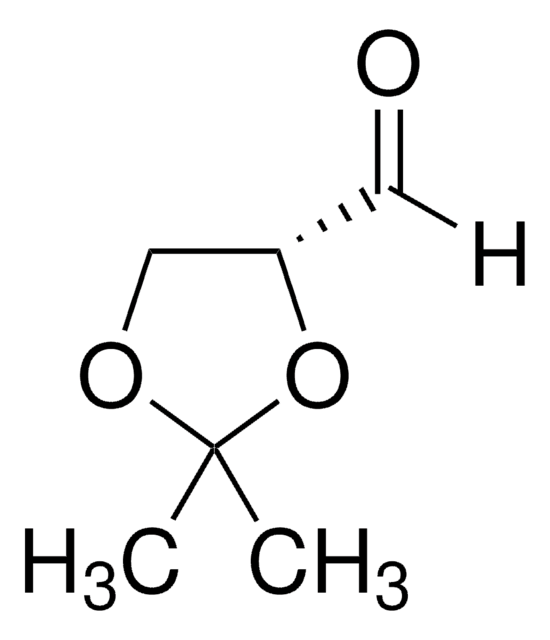
G4802
DL-Glyceraldehyde, dimer
95%
Synonym(s):
3,6-Dihydroxy-1,4-dioxane-2,5-dimethanol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H12O6
CAS Number:
Molecular Weight:
180.16
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
mp
144-145 °C (lit.)
storage temp.
2-8°C
SMILES string
OC[C@H]1O[C@@H](O)[C@H](CO)O[C@@H]1O
InChI
1S/2C3H6O3/c2*4-1-3(6)2-5/h2*1,3,5-6H,2H2
InChI key
NGNVWCSFFIVLAR-UHFFFAOYSA-N
General description
DL-Glyceraldehyde , dimer, a dimeric form of DL-glyceraldehyde is an organic compound that possesses a symmetrical p-dioxan crystalline structure with equatorially bonded hydroxy and hydroxymethyl groups. It reacts with a primary alkyl amine to form a Schiff base, during the synthesis of DL-alanine derivatives.
Application
- Aldehyde Reductase Activity: The enzymatic properties of DL-Glyceraldehyde dimer were examined in the context of aldehyde reductases from Euonymus japonica leaves, providing insights into the reduction processes of aldose sugars, which are significant for understanding stress responses in plants (Negm, 1986).
- Enzymatic Reductase Functions: DL-Glyceraldehyde dimer was studied in the purification and characterization of human liver aldehyde reductases, focusing on its role in metabolic detoxification, crucial for pharmaceutical applications involving drug metabolism and toxicity (Petrash and Srivastava, 1982).
- Protozoan Metabolic Pathways: The enzymatic characterization of aldehyde reductase from Crithidia fasciculata, with DL-Glyceraldehyde dimer as a substrate, provided insights into the metabolic pathways of protozoans, important for developing treatments against parasitic infections (Kobayashi, 1982).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Non-enzymatic transformation of dl-glyceraldehyde, 1, 3-dihydroxyacetone, and pyruvaldehyde with primary amine to the same dl-alanine derivatives
Yande H, et al.
Tetrahedron Letters, 56, 4516-4519 (2015)
Experimental and Theoretical Study of the Products from the Spontaneous Dimerization of DL-and D-Glyceraldehyde
Federico G, et al.
Journal of the Brazilian Chemical Society, 16, 467-476 (2005)
Chan Mee Lee et al.
Archives of pharmacal research, 38(6), 1090-1098 (2014-10-16)
Aldose reductase (AR) is a key enzyme in the polyol pathway that is strongly implicated in the pathogenesis of diabetic complications. AR inhibitors have been proposed as therapeutic agents for diabetic complications through suppression of sorbitol formation and accumulation. In
Jae Sue Choi et al.
Archives of pharmacal research, 37(10), 1354-1363 (2014-07-06)
To investigate the effect of C-glycosylation at different positions of luteolin, the structure-activity relationships of luteolin and a pair of isomeric C-glycosylated derivatives orientin and isoorientin, were evaluated. We investigated the effects of C-glycosylation on the antioxidant, anti-Alzheimer's disease (AD)
Md Nurul Islam et al.
Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association, 69, 55-62 (2014-04-10)
Vicenin 2, isolated from a traditionally used medicinal plant Artemisia capillaris, is a 6,8-di-C-glucoside of apigenin which has been previously reported to possess a wide variety of pharmacological activities including antioxidant, anti-inflammatory, anti-cancer, and hepatoprotective. However, there have not been
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service