921459
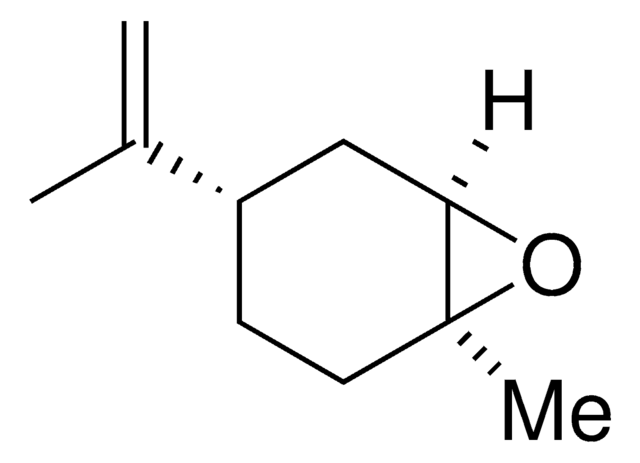
trans-(+)-limonene oxide
Synonym(s):
(1S,4R,6R)-4-Isopropenyl-1-methyl-7-oxabicyclo[4.1.0]heptane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H16O
CAS Number:
Molecular Weight:
152.23
MDL number:
UNSPSC Code:
12352212
NACRES:
NA.22
Recommended Products
form
liquid
Quality Level
InChI
1S/C10H16O/c1-7(2)8-4-5-10(3)9(6-8)11-10/h8-9H,1,4-6H2,2-3H3/t8-,9-,10+/m1/s1
InChI key
CCEFMUBVSUDRLG-BBBLOLIVSA-N
Application
trans-(+)-Limonene oxide is a valuable chiral terpene oxide building block. Used in the synthesis of Phosphorus Incorporation (PI) reagents, as a biologically sourced component of polycarbonate polymers, and as a building block for synthetic applications requiring a reactive epoxide or alkene.
related product
Product No.
Description
Pricing
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
149.0 °F - closed cup
Flash Point(C)
65 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Ke-Yin Ye et al.
Journal of the American Chemical Society, 141(24), 9548-9554 (2019-06-11)
Organic radicals are generally short-lived intermediates with exceptionally high reactivity. Strategically, achieving synthetically useful transformations mediated by organic radicals requires both efficient initiation and selective termination events. Here, we report a new catalytic strategy, namely, bimetallic radical redox-relay, in the
Synthesis and self-assembly of biobased poly(limonene carbonate)-block-poly(cyclohexene carbonate) diblock copolymers prepared by sequential ring-opening copolymerization.
Bailer J, et al.
Green Chemistry, 21, 2266-2272 (2019)
Samantha A Green et al.
Journal of the American Chemical Society, 141(19), 7709-7714 (2019-04-30)
Metal-hydride hydrogen atom transfer (MHAT) functionalizes alkenes with predictable branched (Markovnikov) selectivity. The breadth of these transformations has been confined to π-radical traps; no sp3 electrophiles have been reported. Here we describe a Mn/Ni dual catalytic system that hydroalkylates unactivated
Julian C Lo et al.
Journal of the American Chemical Society, 139(6), 2484-2503 (2017-01-18)
This Article details the development of the iron-catalyzed conversion of olefins to radicals and their subsequent use in the construction of C-C bonds. Optimization of a reductive diene cyclization led to the development of an intermolecular cross-coupling of electronically-differentiated donor
Dongmin Xu et al.
Journal of the American Chemical Society, 142(12), 5785-5792 (2020-02-29)
Phosphorus Incorporation (PI, abbreviated Π) reagents for the modular, scalable, and stereospecific synthesis of chiral phosphines and methylphosphonate nucleotides are reported. Synthesized from trans-limonene oxide, this reagent class displays an unexpected reactivity profile and enables access to chemical space distinct
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service